Abstract
Polypyrrole nanofibers/meso-tetra(4-sulfonatophenyl)porphyrin/graphene quantum dots nanohybrid (PTG) was synthesized by a facile chemical oxidative reaction using persulfate salt of cetyltrimethylammonium cation ((CTA)2S2O8) as the oxidative template. The facile formation and interactions among different constituents were established by FT-IR, UV–Vis, XRD and XPS studies. Morphological analysis was carried out by SEM and HRTEM showing polypyrrole nanofibers (PNF) to be formed with diameters in the range of 29–57 nm and HRTEM micrographs depicted the intercalation of meso-tetra(4-sulfonatophenyl)porphyrin (TSPP) and graphene quantum dots (GQDs) with PNF. The electrical conductivity of PNF and PTG was evaluated using four-probe conductivity measurement. Electrochemical activity of PNF and PTG deposited on indium tin oxide (ITO) electrode was demonstrated using cyclic voltammetry with Fe(CN) 4−/3−6 as the redox species. PTG electrode displayed an enhanced voltammetric response vis-à-vis PNF due to the synergistic effect of PNF, TSPP and GQDs resulting in an increased electroactive area beneficial for a sensing probe. Further, the PTG electrode was checked for its capability to simultaneously detect Cd2+ and Pb2+ in aqueous solution by differential pulse voltammetry (DPV).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypyrrole (ppy), a conducting polymer, has risen as a multifunctional material since its discovery in the early twentieth century [1] with the current research being focussed on it. It is the preferred choice among conducting polymers due to its ease of synthesis both chemically and electrochemically, appreciable redox electroactivity, good environmental stability, non-toxicity, biocompatibility and low cost [2,3,4,5]. It has wide range of applications in supercapacitors and energy storage devices [6,7,8], biosensors [9,10,11], chemical sensors [12, 13], medical field [14], solar cells [15], catalysis [16] and as an adsorbent for metal ions [17]. Its facile synthesis in both aqueous and non-aqueous solvents with flexibility of pH adjustment that can be acidic or neutral gives the opportunity to synthesize a variety of composites and hybrids with carbonaceous materials such as graphene [7, 8, 18], GQDs [9], carbon nanotubes [15], phthalocyanines [19, 20] and porphyrins [21,22,23,24,25,26]. Porphyrin, a stable macrocycle [27], has been used in diverse fields such as solar cells, photodynamic therapy, catalysis and electrochemical sensors due to its metal coordinating property, π-conjugated structure, unique optical and redox properties. Fan et al. [28] have synthesized zinc(II)-porphyrin derivatives for non-enzymatic H2O2 electrochemical sensing. Paul et al. [22] incorporated 5,10,15,20-tetraphenyl-21H,23H-porphyrin iron(III) chloride into polypyrrole and developed CO sensor. Zhou et al. [26] prepared electrocatalyst for oxygen reduction from nanocomposites of cobalt porphyrin and polypyrrole. Recently, polypyrrole/hemin nanocomposite was synthesized by Hu et al. [21] with applications as a glucose biosensor, in dye removal and photothermal therapy. Buntem et al. [29] used porphyrin with silica for metal ion sensing, whereas Zhang et al. [30] developed ultrasensitive Cu2+ sensors using meso-tetra(4-sulfonatophenyl)porphyrin. On the other hand, the zero-dimensional GQDs with their quantum confinement and edge effects render them with unique electrical and optical properties. Additionally due to their electron-rich sp2 hybridized carbon skeleton, abundant oxygen-containing functional groups and low toxicity, they have gained importance in areas such as electrochemical sensors, photovoltaic devices, catalysis and bioimaging [31,32,33]. Chen et al. [34] prepared GQD-doped polypyrrole for use as counter electrodes in dye sensitized solar cells. Wang et al. [9] used ppy, GQDs and Prussian Blue to make a nanocomposite for electrochemical sensing of l-cysteine. GQDs have also been used in metal ion sensing like in the works reported by Ting et al. [31] and Qian et al. [32] for determination of heavy metal ions including Hg2+ and Cu2+ by the former and Pb2+ by the latter. Metal ion sensors especially for heavy, toxic metal ions are necessary because heavy metal pollution is a growing environmental problem affecting living beings both on land and on water [35, 36]. It is considered a serious threat because these pollutants are highly toxic even at low concentration and are non-biodegradable [37] with allowed values of 3 and 10 ppb for Cd2+ and Pb2+, respectively, as given by the World Health Organization (WHO) [38]. Thus, in this work, it was considered of interest to develop a nanohybrid-containing ppy, TSPP and GQDs based on their above advantages and its characterization, which could be applied in future works for easy and simultaneous electrochemical/optical detection of Cd2+ and Pb2+. The interaction between nitrogen of ppy and metal ions improves its prospects to be used for their detection [39]. Polypyrrole was used in the form of nanofibers which were formed using cetyltrimethylammonium bromide (CTAB) as template, and it was found that morphology had enormous impact on its redox properties as nanofibers provided a higher surface-to-volume ratio as compared to polypyrrole microspheres. An anionic water-soluble porphyrin namely tetrasodium salt of meso-tetra(4-sulfonatophenyl)porphyrin (TSPP) was used which simultaneously doped the PNF through its sulfonate groups and also provided a core for binding of metal ions.
Experimental
Materials and methods
Pyrrole monomer (99%, Spectrochem) was distilled before use, tetrasodium salt of meso-tetra(4-sulfonatophenyl)porphyrin (TSPP) was purchased from Sigma-Aldrich (≥ 98.0%) and other chemicals—cadmium(II) chloride monohydrate, CdCl2.H2O (CDH), lead(II) acetate trihydrate, Pb(CH3COO)2.3H2O (CDH), copper(II) acetate monohydrate, Cu(CH3COO)2.H2O (Fisher Scientific), zinc(II) acetate dihydrate, Zn(CH3COO)2.2H2O (CDH), nickel(II) chloride hexahydrate, NiCl2.6H2O (S.D. Fine Chem. Limited), ammonium persulfate, (NH4)2S2O8 (APS) (CDH), sodium hydroxide, NaOH (Fisher Scientific), citric acid anhydrous (CA) (Merck), sodium acetate trihydrate, CH3COONa.3H2O (Fisher Scientific), acetic acid glacial, CH3COOH (SRL), cetyltrimethylammonium bromide (CTAB, Spectrochem), potassium ferrocyanide, K4Fe(CN)6.3H2O (CDH), potassium ferricyanide, K3Fe(CN)6 (SRL)—were of AR grade and used as such. Nafion D-520 dispersion (5% w/w in water and 1-propanol, Alfa Aesar) and absolute ethanol C2H5OH were also used without any further purification. Deionized water with resistivity of 18.2 MΩ cm at 25 °C was used for all experiments. 10 mM stock solutions of Cd2+ and Pb2+ was used for making further dilutions.
Preparation of polypyrrole nanofibers (PNF)
PNF were prepared according to an earlier reported method [40]. A homogeneous aqueous solution of the cationic surfactant CTAB (30 ml, 0.01 M) was prepared by stirring the surfactant in water. Pyrrole monomer (0.03 M) was added to it and was vigorously stirred for 10 min so that the pyrrole molecules could arrange themselves inside the hydrophobic cavity of the surfactant micelles. It was cooled to around 5–8 °C in an ice bath. Thereafter, a pre-cooled solution of APS (0.14 M, 6.5 mL) was added to it drop-wise along with vigorous stirring. It generated a white precipitate on account of formation of the persulfate salt of the cationic surfactant that acted as the oxidizing template. A gradual color change to black was observed with progress in the polymerization of pyrrole. After that, the reaction mixture was kept undisturbed at 0 °C for 24 h which was later centrifuged to separate the solid product (PNF). It was washed with deionized water and ethanol to remove any remaining surfactant and unreacted reactants. The collected PNF were dried in oven at 60 °C overnight.
Preparation of graphene quantum dots (GQDs)
Blue luminescent GQDs were prepared by a similar procedure as reported earlier [41] in which citric acid was directly pyrolyzed. 2 g of citric acid (anhydrous) was taken in a 50-mL beaker and heated at 200–220 °C in an oil bath for 30 min. After 4 min of heating, the white powder completely melted with a gradual color change from colorless to light yellow and finally orange indicating the formation of GQDs. The viscous orange liquid was added drop-wise to NaOH aqueous solution (0.25 M, 50 mL) with vigorous stirring resulting in a golden yellow-colored solution with pH 5. It was neutralized to pH 7 with further addition of NaOH solution and stored at 0–4 °C. It was also observed that the obtained aqueous solution when viewed under UV light showed blue luminescence. Digital photographs for the above steps are given in Fig. S1.
Preparation of meso-tetra(4-sulfonatophenyl)porphyrin-doped polypyrrole nanofibers with GQDs (PTG)
PTG nanohybrid was prepared in situ during the preparation of PNF. After the addition of pyrrole monomer (0.03 M) in 30 mL of homogeneous solution of CTAB (0.01 M) and vigorous stirring of 10 min, TSPP (1 mM) was added which changed the color of the solution to reddish purple from colorless. Subsequently, GQDs solution was injected (3:1 v/v with respect to pyrrole) into it with a 10-min interval between the additions. The reaction mixture was cooled to 5–8 °C followed by drop-wise addition of pre-cooled solution of APS (0.14 M, 6.5 mL). The color of the reaction mixture changed to a bright green color which gradually changed to black with hints of green. The reaction mixture was kept undisturbed at 0 °C for 24 h after which it was centrifuged and washed with deionized water and ethanol. The collected product was dried in oven at 60 °C overnight.
Electrode preparation and electrochemical measurements
The construction of PNF and PTG electrodes was performed as follows: 20 μL of the uniform suspension made by ultrasonicating a mixture of the sample (5 mg), Nafion (5 μL) and isopropanol was drop-casted on a 1 cm × 3 cm ITO electrode. The ITO electrode prior to use was ultrasonically cleaned in a stepwise manner, first in soap solution, then in deionized water and finally with acetone. Electrochemical measurements were done by cyclic voltammetry and differential pulse voltammetry (DPV) in a three-electrode cell assembly powered by a potentiostat (CorrTest two channel electrochemical workstation) using ITO, PNF and PTG electrodes, Ag/AgCl (3 M KCl) and platinum wire as the working, reference and counter electrodes, respectively. The electrolyte used was 1 mM Fe(CN) 4−/3−6 (aq) with 0.1 M KCl as the supporting electrolyte and acetic acid–sodium acetate buffer (HAc-NaAc, pH 5) for different experiments. The simultaneous detection of Cd2+ and Pb2+ (6 μM) was checked by DPV in HAc-NaAc buffer (pH 5) at a potential window of − 1.0 to − 0.3 V, pulse amplitude of 0.050 V with increment potential of 0.004 V, pulse width of 0.03 s and pulse period of 0.2 s.
Characterization
The morphological studies of PNF and PTG were carried out using scanning electron microscopy (SEM) on SEM ZEISS EVO18 at a voltage of 20 kV and high-resolution transmission electron microscopy (HRTEM) on FEI Tecnai G2 F30 at 300 kV. FT-IR spectra were recorded using Shimadzu IRAffinity-1S in KBr pellet mode with 45 scans at a resolution of 4 cm−1 from 4000 to 400 cm−1. UV–Vis spectra were obtained on a Hitachi U-2900 UV–visible spectrophotometer in water. X-ray diffraction (XRD) was carried on a Rigaku Ultima IV X-ray diffractometer with Cu Kα radiation (λ = 1.541 Å) at an operating voltage and current of 40 kV and 30 mA, respectively. X-ray photoelectron spectroscopy (XPS) was performed using Al Kα (1486.71 eV) X-ray radiation. Electrical conductivity was measured by four-probe method using Keithley 2450.
Results and discussions
Formation mechanism of PNF and PTG
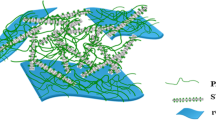
Polypyrrole nanofibers (PNF) were prepared using an oxidative template comprising of cationic surfactant, CTAB and APS [40]; as without it, literature studies have shown the formation of polypyrrole mostly in the form of spheres [42, 43]. CTAB aqueous solution which was taken at a higher concentration than its cmc (0.91 mM) [44] formed micelles in solution with hydrophobic alkyl chains in the interior and polar head groups in contact with the surrounding water molecules. With the addition of pyrrole, an organic liquid, it arranged itself in the interior cavity of the micelles on account of the hydrophobic effect. APS was added then, which resulted in the formation of a white precipitate of (CTA)2S2O8. The S2O8 2− ions led to the formation of lamellar ionic micelles acting as a binding force between the lamellae. With the progress in polymerization by (CTA)2S2O8, the oxidative template, lamellar polypyrrole was prepared, but the degradation of persulfate ions led to the weakening and disappearance of bonding between the lamellae causing an increase in the surface/interface energy. In order to lower this energy, the lamellae curled and rolled [45] leading to the formation of PNF. PTG was formed by the addition of TSPP and GQDs into the surfactant solution along with pyrrole. When TSPP, an anionic porphyrin, was added to the CTAB aqueous solution, the color of the reaction mixture turned to a reddish purple color signifying it to be present in the non-aggregated monomer form inside the micelles or dissolution of TSPP took place in the micelles. This phenomenon occurred as [CTAB] was well above its critical micellar concentration (cmc). Subsequent addition of APS brought about a color change to bright green which generally occurs when porphyrin is present in the form of lamellar J-aggregates at a low pH, but here it was mildly acidic (pH ~ 5–6); hence, this aggregation was assumed to occur in the form of π–π interactions, H-bonding and electrostatic interactions with GQDs and PNF. Based on the above mechanism, a schematic illustration for the preparation of PTG is displayed in Fig. 1.
Analysis by FT-IR, UV–Vis spectroscopy and X-ray diffraction (XRD)
Chemical structure of PNF and PTG was determined by FT-IR spectroscopy. Figure 2 shows the FT-IR spectra of PNF and PTG. FT-IR spectrum of PNF depicted all the characteristic peaks of polypyrrole as reported in the literature [40, 46]. The medium broadband at 3500–3000 cm−1 was ascribed to N–H and aromatic C–H stretching vibrations. The peaks observed at 1548 and 1479 cm−1 were assigned to antisymmetric and symmetric ring stretching modes of polypyrrole, respectively. Peaks at 1313 and 1043 cm−1 were attributed to C–N stretching and C–H in-plane deformation vibration denoting 2,5-disubstituted pyrrole, respectively. Furthermore, the two strong peaks observed at 1192 and 914 cm−1 represented the doping state of ppy. The bands observed at 964 and 786 cm−1 established the formation of polymerized pyrrole corresponding to C–C and C–H out-of-plane ring deformation, respectively. In the FT-IR spectrum of PTG, all the constituents—GQDs, TSPP and PNF—were clearly recognized suggesting the successful formation of the hybrid and doping of PNF by GQDs and \({\text{SO}_{3}^-}\) groups of TSPP. However, compared to PNF, the majority of characteristic peaks showed an upward shift to high wavenumbers viz. 1556 cm−1 (1548 cm−1), 1485 cm−1 (1479 cm−1), 1317 cm−1 (1313 cm−1), 1049 cm−1 (1043 cm−1), 966 cm−1 (964 cm−1) and 923 cm−1 (914 cm−1) as a consequence of homogeneous effect of TSPP and GQDs on PNF. Hydroxyl and carboxyl groups of GQDs were present at 3122 and 1712 cm−1, respectively, while TSPP’s presence was verified by N–H stretching vibration at 3471 cm−1, a shoulder at 1643 cm−1 for aromatic C=C stretching, peaks at 1188 and 1112 cm−1 assigned to νasymm \(({\text{SO}_{3}^-})\) and νsymm \(({\text{SO}_{3}^-})\) vibrations [47, 48], weak peaks at 800 and 734 cm−1 ascribed to C−H out-of-plane bending vibration for 1,4-disubstituted phenyl ring and S–O stretching vibration, respectively [49]. It was concluded from FT-IR examination that the phenyl and \({\text{SO}_{3}^-}\) groups of TSPP were involved in hybrid formation due to the presence of a shoulder at 1643 cm−1 assigned to aromatic C=C stretching which was observed as a strong peak in TSPP (Fig. S2) at 1641 cm−1 indicating π–π interaction between TSPP, pyrrole rings in PNF and GQDs planes. Also, porphyrin ring stretching modes of C α –C β , C β –C β , C α –C m (m-mesocarbon) at 1550 and 1469 cm−1 [50] were absent that implied conformational change in the porphyrin ring due to interaction of mesosubstituents with other constituents. Besides, the peaks for νasymm \(({\text{SO}_{3}^-})\) and νsymm \(({\text{SO}_{3}^-})\) vibrations weakened and shifted to lower wavenumbers as compared to original TSPP (1193 and 1124 cm−1) suggesting the involvement of sulfonate group in electrostatic interaction with polypyrrole backbone. Furthermore, peaks at 800 and 734 cm−1 ascribed to C–H out-of-plane bending vibration for 1,4-disubstituted phenyl ring and S–O stretching vibration, respectively, appeared as weak peaks and shifted to lower wavenumbers when compared with TSPP (804 and 738 cm−1). Another striking observation was the absence of a strong peak at 636 cm−1 which was present in TSPP depicting C–S stretching vibration. All these corroborated the fact that phenyl and \({\text{SO}_{3}^-}\) groups of TSPP were involved in hybrid formation. The FT-IR spectrum for GQDs is given in Fig. S3.
The UV–Vis absorption spectra of PNF and PTG are shown in Fig. 3. PNF (Fig. 3a) depicted the characteristic absorption spectrum for ppy. The absorption band at about 430 nm was ascribed to the π → π* transition of ppy chains and the broadband beyond 600 nm extending toward the near infrared region was due to the transitions from valence band to polaron/bipolaron/antibonding bipolaron band signifying doped PNF which was consistent with earlier reported works [51, 52]. PTG (Fig. 3b) showed three prominent peaks at 424, 489 and 707 nm. The Soret band of TSPP bathochromically shifted and also showed hypochromicity with the emergence of new bands at 489 and 707-nm vis-à-vis TSPP (Fig. S4) [53] signaling the association of porphyrin monomers with GQDs and oxidized PNF. Literature studies revealed that these peaks corresponded to the formation of J-aggregates of TSPP under strong acidic conditions [47, 53, 54], but in the present work the conditions were only slightly acidic with pH ~ 5–6; hence, it was concluded that TSPP was involved in π–π interactions with GQDs and PNF and also electrostatic interactions with positively charged PNF backbone through \({\text{SO}_{3}^-}\) groups resulting in a similar effect as J-aggregation. Thus, PTG nanohybrid was successfully formed as also observed in FT-IR results. The UV–Vis spectrum of GQDs is presented in Fig. S5.
XRD pattern of PNF (Fig. 4a) showed a broad amorphous peak centered at 2θ = 23.6o with d-spacing of 3.77 Å attributed to the scattering from polypyrrole chains in PNF at interplanar distance as reported in the literature [55,56,57]. PTG (Fig. 4b) depicted a similar diffractogram to that of PNF with the broad peak centered at 2θ = 23.5o with increased intensity denoting that no extra crystalline phase has been formed in the hybrid with uniform distribution of TSPP and GQDs.
Morphological studies by SEM and HRTEM
SEM micrographs of PNF (Fig. 5a) represented noodle-like morphology. The diameters of the nanofibers formed were in the range of 29–57 nm. The surface morphology of PTG (Fig. 5b) also showed fibrous structure with the constituents, viz. TSPP and GQDs covering and aggregating on the nanofibers. HRTEM image of PTG (Fig. 5c, d) gave a clearer description about the hybrid formation with the PNF being intercalated by TSPP and GQDs nanosheets. These results further endorsed earlier results of FT-IR and UV–Vis spectroscopy that PNF, TSPP and GQDs successfully formed the hybrid by interacting with each other through π–π and electrostatic interactions.
X-ray photoelectron spectroscopy (XPS) analysis
Chemical composition of PTG was determined by X-ray photoelectron spectroscopy (XPS). The survey spectrum (Fig. 6a) showed the presence of C, N, O and S elements with peaks corresponding to C 1s, N 1s, O 1s and S 2p at their characteristic binding energies [58]. C 1s, N 1s and O 1s peaks were also studied by their respective high-resolution spectra (Fig. 6b, c, d). The N 1 s peak was deconvoluted into four components representing –NH– in pyrrole, polaron (C–N+), bipolaron (C=N+) and C=N at ~ 399, ~ 400, ~ 402 and ~ 398 eV, respectively. The deconvolution of C 1 s peak resulted in five components attributed to C α and C β of ppy at ~ 284.6 and ~ 283.9 eV, respectively, C–O/C–N, C=O/C=N, O–C=O/N–C=O at around 286, 287.4 and 290 eV, respectively. The high-resolution spectrum of O 1 s peak is depicted to be made up of three components, viz. C–O, C=O and \({\text{SO}_{3}^-}\) at about 531.5, 533.3 and 530.3 eV, respectively. The S 2p peak at ~ 167 eV in the survey spectrum denoted sulfonic acid group of TSPP [59]. Thus, it was demonstrated that TSPP and GQDs had inserted between PNF and the results were consistent with FT-IR study.
Electrochemical behavior of PNF and PTG electrodes
The cyclic voltammograms (CVs) of bare ITO, PNF and PTG electrodes (Fig. 7) were recorded in 1 mM Fe(CN) 4−/3−6 (aq) with 0.1 M KCl as the supporting electrolyte over a potential window of − 1.0 to + 1.0 V at a scan rate of 0.010 V s−1. All the CVs showed distinct oxidation and reduction peaks corresponding to Fe(CN) 4−/3−6 redox couple. The curves had been identified to represent a quasi-reversible electrode reaction with the peak potential difference (ΔEp) and ratio of anodic to cathodic peak current (I pa/I pc) as 90 mV, 1.02 for bare ITO; 110 mV, 1.50 for PNF and 130 mV, 1.60 for PTG electrode. The PNF electrode displayed an enhanced current as compared to bare ITO confirming the successful modification and deposition by a matrix with better conductivity, charge transfer rate and large surface area due to the nanosized effect of PNF. Furthermore, the PTG electrode depicted even better Faradaic redox response than PNF electrode with twofold increase in current, owing to the presence of GQDs and TSPP providing improved electron transport channels. The synergistic structural organization of PNF, GQDs and TSPP in the hybrid also made this achievable.
Moreover, the knowledge about the electron transfer mechanism was obtained by plotting a graph between peak current vs square root of scan rate (Fig. 8a) by recording the cyclic voltammograms of bare ITO (Fig. 8b), PNF (Fig. 8c) and PTG (Fig. 8d) electrodes at different scan rates. It was found that it varied in a linear manner, thus obeying Randles–Sevcik equation as given in Eq. 1, and a diffusion-controlled reaction was inferred. It was then employed to estimate the electroactive surface area of PNF and PTG electrodes utilizing the slope calculated from the graphs [60].
where i p = peak current, n = number of electrons in the redox reaction, ν = scan rate (V s−1), D = diffusion coefficient (5.7 × 10−6 cm2 s−1), A = surface area (cm2) and C = concentration of analyte (mol cm−3). The electroactive surface area of bare ITO, PNF and PTG electrodes was 2.74, 3.83 and 7.75 cm2, respectively. The increase in area proved the modification of ITO electrode by better conductive materials. The electrical conductivity of PNF and PTG was measured by four-probe method and was determined to be 2.75 and 3.15 mS cm−1.
a Plot of peak current and square root of scan rate for (filled square, filled circle) bare ITO, (filled triangle, filled down pointing triangle) PNF and (filled diamond suit, filled left pointing triangle) PTG electrodes and cyclic voltammograms of b bare ITO electrode c PNF electrode and d PTG electrode at different scan rates varying from 0.010 to 0.100 V s−1 in 1 mM Fe(CN) 4−/3−6 and 0.1 M KCl as the supporting electrolyte over a potential window of − 1.0 to + 1.0 V
Detection of Cd2+ and Pb2+ by UV–Vis spectroscopy and DPV
The response of PTG as a sensor for Cd2+ and Pb2+ was initially determined by UV–Vis spectroscopy. A dilute dispersion of PTG (20 mL) prepared in deionized water was mixed with 200 nM of Cd2+ and Pb2+ individually and kept undisturbed for 24 h. The UV–Vis spectra for PTG before and after addition of target analyte metal ions are displayed in Fig. 9. The Soret band at 424 nm of PTG (Fig. 9a) experienced strong hypochromicity along with suppression of the other two peaks at 489 and 707 nm with Cd2+ (Fig. 9b). Similarly in Fig. 9c in the case of Pb2+ the peaks suffered hypochromicity and were also bathochromically shifted. The significant spectral changes indicated coordination of metal ions with the hybrid and causing a change in the electronic environment, hence establishing its ability for detecting Cd2+ and Pb2+.
PTG electrode was used to check its applicability for simultaneous detection of Cd2+ and Pb2+ (6 μM) by DPV technique in 0.1 M HAc-NaAc buffer (pH 5). The effect of pH was studied in the range 4.0–6.0. The dependence of DPV peak current on pH was observed with the maximum current being obtained at pH 5. The current decreased for 5 > pH > 5 which was attributed to the protonation of a few binding sites for metal cations in the hybrid and lesser availability of metal cations due to their chemical speciation reaction resulting in the formation of hydroxides in the latter condition. The voltammogram (Fig. 10) clearly depicted the presence of two anodic peaks at − 0.67 and − 0.48 V for Cd2+ and Pb2+, respectively, thus demonstrating simultaneous detection. Interference studies were also investigated with tenfold addition of Ni2+, Zn2+ and Cu2+ with respect to Cd2+ and Pb2+, whereby it was noticed that Ni2+ presented no interference. However, Zn2+ and Cu2+ slightly interfered with Pb2+ and the peak current moderately increased.
Conclusions
The aim of the work to prepare the nanohybrid utilizing polypyrrole, TSPP and GQDs was successfully achieved which was confirmed by various analytical techniques such as FT-IR and XPS. Cyclic voltammetry analysis revealed that the nanosized form of polypyrrole in the form of nanofibers greatly enhanced the electrochemical response which further increased on incorporation of GQDs and TSPP, thus providing better channel for electron transfer. HRTEM investigation of PTG showed PNF being intercalated by TSPP and GQDs nanosheets. UV–Vis spectroscopy showed that the hybrid was able to interact strongly with Cd2+ and Pb2+ which was also checked electrochemically by DPV. Overall, it presented an easy and cost-effective method which could be explored for detection of Cd2+ and Pb2+.
References
Nalwa HS (2001) Handbook of advanced electronic and photonic materials and devices: semiconductors, vol 1. Academic Press, San Diego
Husson J, Lakard S, Monney S, Buron CC, Lakard B (2016) Elaboration and characterization of carboxylic acid-functionalized polypyrrole films. Synth Met 220:247–254
Schirmer KSU, Esrafilzadeh D, Thompson BC, Quigley AF, Kapsa RMI, Wallace GG (2016) Conductive composite fibres from reduced graphene oxide and polypyrrole nanoparticles. J Mater Chem B 4:1142–1149
Baleviciute I, Ratautaite V, Ramanaviciene A, Balevicius Z, Broeders J, Croux D, McDonald M, Vahidpour F, Thoelen R, Ceuninck WD, Haenen K, Nesladek M, Reza A, Ramanavicius A (2015) Evaluation of theophylline imprinted polypyrrole film. Synth Met 209:206–211
Ramaprasad AT, Latha D, Rao V (2017) Synthesis and characterization of polypyrrole grafted chitin. J Phys Chem Solids 104:169–174
Thakur VK, Kessler MR (2017) In: A. Tiwari, S. Valyukh (eds) Polymer nanocomposites: new advanced dielectric materials for energy storage applications, in advanced energy materials. John Wiley & Sons, Inc., Hoboken, p. 207
Atri P, Tiwari DC, Sharma R (2017) Synthesis of reduced graphene oxide nanoscrolls embedded in polypyrrole matrix for supercapacitor applications. Synth Met 227:21–28
Sačer D, Čapeta D, Šrut Rakić I, Peter R, Petravić M, Kraljić Roković M (2016) Tailoring polypyrrole supercapacitive properties by intercalation of graphene oxide within the layer. Electrochim Acta 193:311–320
Wang L, Tricard S, Yue P, Zhao J, Fang J, Shen W (2016) Polypyrrole and graphene quantum dots @ Prussian Blue hybrid film on graphite felt electrodes: Application for amperometric determination of L-cysteine. Biosens Bioelectron 77:1112–1118
Sharma A, Kumar A (2016) Study of structural and electro-catalytic behaviour of amperometric biosensor based on chitosan/polypyrrole nanotubes-gold nanoparticles nanocomposites. Synth Met 220:551–559
Nia PM, Meng WP, Lorestani F, Mahmoudian MR, Alias Y (2015) Electrodeposition of copper oxide/polypyrrole/reduced graphene oxide as a nonenzymatic glucose biosensor. Sens Actuators B Chem 209:100–108
Kaushik A, Kumar R, Arya SK, Nair M, Malhotra BD, Bhansali S (2015) Organic-inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem Rev 115:4571–4606
Mahmoudian MR, Basirun WJ, Alias Y (2016) A sensitive electrochemical Hg2+ ions sensor based on polypyrrole coated nanospherical platinum. RSC Adv 6:36459–36466
Sharma M, Waterhouse GIN, Loader SWC, Garg S, Svirskis D (2013) High surface area polypyrrole scaffolds for tunable drug delivery. Int J Pharm 443:163–168
Wang W-Y, Ting P-N, Lo C-S, Lin J-Y (2014) Pulse-reversal electropolymerization of polypyrrole on functionalized carbon nanotubes as composite counter electrodes in dye-sensitized solar cells. Electrochim Acta 137:721–727
Kumar GG, Kirubaharan CJ, Udhayakumar S, Ramachandran K, Karthikeyan C, Renganathan R, Nahm KS (2014) Synthesis, structural, and morphological characterizations of reduced graphene oxide-supported polypyrrole anode catalysts for improved microbial fuel cell performances. ACS Sustain Chem Eng 2:2283–2290
Ekramul Mahmud HNM, Huq AKO, Yahya RB (2016) The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. RSC Adv 6:14778–14791
Bora C, Sharma J, Dolui SK (2014) Polypyrrole/Sulfonated graphene composite as electrode material for supercapacitor. J Phys Chem C 118:29688–29694
Porras-Gutiérrez AG, Frontana-Uribe BA, Gutiérrez-Granados S, Griveau S, Bedioui F (2013) In situ characterization by cyclic voltammetry and conductance of composites based on polypyrrole, multi-walled carbon nanotubes and cobalt phthalocyanine. Electrochim Acta 89:840–847
Patois T, Sanchez JB, Berger F, Fievet P, Segut O, Moutarlier V, Bouvet M, Lakard B (2013) Elaboration of ammonia gas sensors based on electrodeposited polypyrrole-cobalt phthalocyanine hybrid films. Talanta 117:45–54
Hu P, Han L, Dong S (2014) A facile one-pot method to synthesize a polypyrrole/hemin nanocomposite and its application in biosensor, dye removal, and photothermal therapy. ACS Appl Mater Interfaces 6:500–506
Paul S, Amalraj F, Radhakrishnan S (2009) CO sensor based on polypyrrole functionalized with iron porphyrin. Synth Met 159:1019–1023
Zhang W, Chen J, Wagner P, Swiegers GF, Wallace GG (2008) Polypyrrole/Co-tetraphenylporphyrin modified carbon fibre paper as a fuel cell electrocatalyst of oxygen reduction. Electrochem Commun 10:519–522
Diab N, Oni J, Schulte A, Radtke I, Blochl A, Schuhmann W (2003) Electropolymerized manganese porphyrin/polypyrrole films as catalytic surfaces for the oxidation of nitric oxide. Talanta 61:43–51
De Gregori I, Carrier M, Deronzier A, Moutet JC, Bedioui F, Devynck J (1992) Incorporation of anionic cobalt porphyrin by anion exchange into polypyrrole films containing alkylammonium groups. J Chem Soc Faraday Trans 88:1567–1572
Zhou Q, Li CM, Li J, Cui X, Gervasio D (2007) Template-synthesized cobalt Porphyrin/Polypyrrole nanocomposite and its electrocatalysis for oxygen reduction in neutral medium. J Phys Chem C 111:11216–11222
Makiura R, Usui R, Pohl E, Prassides K (2014) Porphyrin-based coordination polymer composed of layered pillarless two-dimensional networks. Chem Lett 43:1161–1163
Fan S, Zhu Y, Liu R, Zhang H, Wang Z-S, Wu H (2016) A porphyrin derivative for the fabrication of highly stable and sensitive electrochemical sensor and its analytical applications. Sens Actuators B Chem 233:206–213
Buntem R, Intasiri A, Lueangchaichaweng W (2010) Facile synthesis of silica monolith doped with meso-tetra(p-carboxyphenyl)-porphyrin as a novel metal ion sensor. J Coll Interface Sci 347:8–14
Zhang J, Devaramani S, Shan D, Lu X (2016) Electrochemiluminescence behavior of meso-tetra(4-sulfonatophenyl)porphyrin in aqueous medium: its application for highly selective sensing of nanomolar Cu2+. Anal Bioanal Chem 408:7155–7163
Ting SL, Ee SJ, Ananthanarayanan A, Leong KC, Chen P (2015) Graphene quantum dots functionalized gold nanoparticles for sensitive electrochemical detection of heavy metal ions. Electrochim Acta 172:7–11
Qian ZS, Shan XY, Chai LJ, Chen JR, Feng H (2015) A fluorescent nanosensor based on graphene quantum dots–aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens Bioelectron 68:225–231
Chua CK, Sofer Z, Simek P, Jankovský O, Klímová K, Bakardjieva S, Kučková SH, Pumera M (2015) Synthesis of strongly fluorescent graphene quantum dots by cage-opening buckminsterfullerene. ACS Nano 9:2548–2555
Chen L, Guo CX, Zhang Q, Lei Y, Xie J, Ee S, Guai G, Song Q, Li CM (2013) Graphene quantum-dot-doped polypyrrole counter electrode for high-performance dye-sensitized solar cells. ACS Appl Mater Interfaces 5:2047–2052
Huang H, Chen T, Liu X, Ma H (2014) Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal Chim Acta 852:45–54
Liu H, Zhou X, Shen J, Xing D (2017) Sensitive detection of Hg2+ with switchable electrochemiluminescence luminophore and disposable bipolar electrode. ChemElectroChem 4:1681–1685
Gautam RK, Sharma SK, Mahiya S, Chattopadhyaya MC (2015) Contamination of heavy metals in aquatic media: transport, toxity and technologies for remediation, in: heavy metals in water: presence, removal and safety. The Royal Soc Chem, 1–24
W. H. Organization (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
Arulraj AD, Devasenathipathy R, Chen S-M, Vasantha VS, Wang S-F (2016) Femtomolar detection of mercuric ions using polypyrrole, pectin and graphene nanocomposites modified electrode. J Coll Interface Sci 483:268–274
Zhang X, Zhang J, Liu Z, Robinson C (2004) Inorganic/organic mesostructure directed synthesis of wire/ribbon-like polypyrrole nanostructures. Chem Commun 16:1852–1853
Dong Y, Shao J, Chen C, Li H, Wang R, Chi Y, Lin X, Chen G (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 50:4738–4743
Plausinaitis D, Sinkevicius L, Mikoliunaite L, Plausinaitiene V, Ramanaviciene A, Ramanavicius A (2017) Electrochemical polypyrrole formation from pyrrole ‘adlayer’. Phys Chem Chem Phys 19:1029–1038
Leonavicius K, Ramanaviciene A, Ramanavicius A (2011) Polymerization model for hydrogen peroxide initiated synthesis of polypyrrole nanoparticles. Langmuir 27:10970–10976
Carmona T, Pineiro M, Monteiro CJP, Pereira MM, Valente AJM (2015) Interactions between cationic surfactants and 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin tetrasodium salt as seen by electric conductometry and spectroscopic techniques. Coll Surf A Physicochem Eng Asp 481:288–296
Shi LY, Wang JQ, Gao L, Huang L, Zhu J, Wang Y, Fan X, Yu T, Zhu M, Li Z, Zou Z (2007) Preparation of mesostructured lamellar zirconia. Mater Manuf Process 22:705–709
Cai Y, Qin Z, Chen L (2011) Effect of electrolytes on electrochemical properties of graphene sheet covered with polypyrrole thin layer. Prog Nat Sci Mater Int 21:460–466
Synytsya A, Synytsya A, Blafková P, Ederová J, Spěvaček J, Slepička P, Král V, Volka K (2009) pH-controlled self-assembling of meso-tetrakis(4-sulfonatophenyl)porphyrin-chitosan complexes. Biomacromolecules 10:1067–1076
Guého CT, Halma M, Charradi K, Forano C, Mousty C (2011) Structural and electrochemical characterization of metallo-porphyrins intercalated into ZnCr-layered double hydroxides: some evidence of dimer formation. New J Chem 35:1898–1905
Khalid M, Acuña JJS, Tumelero MA, Fischer JA, Zoldan VC, Pasa AA (2012) Sulfonated porphyrin doped polyaniline nanotubes and nanofibers: synthesis and characterization. J Mater Chem 22:11340–11346
Hayashi K, Nakamura M, Ishimura K (2012) Silica-porphyrin hybrid nanotubes for in vivo cell tracking by near-infrared fluorescence imaging. Chem Commun 48:3830–3832
Singh A, Salmi Z, Jha P, Joshi N, Kumar A, Decorse P, Lecoq H, Lau-Truong S, Aswal DK, Gupta SK, Chehimi MM (2013) One step synthesis of highly ordered free standing flexible polypyrrole-silver nanocomposite films at air-water interface by photopolymerization. RSC Adv 3:13329–13336
Wang X, Wang T, Liu D, Guo J, Liu P (2016) Synthesis and electrochemical performance of CeO2/PPy nanocomposites: interfacial effect. Ind Eng Chem Res 55:866–874
Biswas S, Ahn H-Y, Bondar MV, Belfield KD (2012) Two-photon absorption enhancement of polymer-templated porphyrin-based J-aggregates. Langmuir 28:1515–1522
Snitka V, Rackaitis M, Rodaite R (2005) Assemblies of TPPS4 porphyrin investigated by TEM, SPM and UV–vis spectroscopy. Sens Actuators B Chem 109:159–166
Liu A, Li C, Bai H, Shi G (2010) Electrochemical deposition of polypyrrole/sulfonated graphene composite films. J Phys Chem C 114:22783–22789
Fu Y, Su Y-S, Manthiram A (2012) Sulfur-polypyrrole composite cathodes for lithium-sulfur batteries. J Electrochem Soc 159:A1420–A1424
Bruck AM, Gannett CN, Bock DC, Smith PF, Marschilok AC, Takeuchi KJ, Takeuchi ES (2017) The electrochemistry of Fe3O4/polypyrrole composite electrodes in lithium-ion cells: the role of polypyrrole in capacity retention. J Electrochem Soc 164:A6260–A6267
Tabačiarová J, Mičušík M, Fedorko P, Omastová M (2015) Study of polypyrrole aging by XPS, FTIR and conductivity measurements. Polym Degrad Stab 120:392–401
Kumar GG, Kim P, Kim AR, Nahm KS, Elizabeth RN (2009) Elizabeth, Structural, thermal and ion transport studies of different particle size nanocomposite fillers incorporated PVdF-HFP hybrid membranes. Mater Chem Phys 115:40–46
Yu C, Ma P, Zhou X, Wang A, Qian T, Wu S, Chen Q (2014) All-Solid-State flexible supercapacitors based on highly dispersed polypyrrole nanowire and reduced graphene oxide composites. ACS Appl Mater Interfaces 6:17937–17943
Acknowledgements
The author (Shruti Peshoria) would like to express gratitude to Guru Gobind Singh Indraprastha University for providing financial support in terms of Indraprastha Research Fellowship (IPRF) with award number GGSIPU/DRC/Ph.D/Adm./2014/1634 and Ms. Neeru Sharma for administrative services. Also, the authors would like to thank Jamia Millia Islamia, New Delhi, for HRTEM and XRD facility and MNIT (Jaipur) for XPS facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2017_1769_MOESM1_ESM.doc
Supplementary data associated with this article including digital photographs of preparation of GQDs, FT-IR and UV–Vis spectra of TSPP and GQDs can be found in the online version. (DOC 2538 kb)
Rights and permissions
About this article
Cite this article
Peshoria, S., Narula, A.K. Structural, morphological and electrochemical properties of a polypyrrole nanohybrid produced by template-assisted fabrication. J Mater Sci 53, 3876–3888 (2018). https://doi.org/10.1007/s10853-017-1769-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1769-z