Abstract
In this project, TiO2@ZnO nanoparticles core–shell nanostructured and titanium dioxide@ mesoporous zinc oxide–graphene oxide (TiO2@ZnO–GO) hybrid nanocomposites as controlled targeted drug delivery systems were synthesized by a facile sono-chemical method. We prepared a novel mesoporous and core–shell structure as a drug nanocarrier (NCs) for the loading and pH-responsive characteristics of the chemotherapeutic curcumin. The structure, surface charge, and surface morphology of NCs were studied using with X-ray diffraction, Fourier transform infrared spectroscopy, dynamic light scattering, brunauer–emmett–teller, scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The SEM and TEM images of NCs show the uniform hexagonal mesoporous morphology with average grain size of about ∼ 190 nm. The drug loading was very high about 16 and 19 for TiO2@ZnO and TiO2@ZnO–GO, respectively. The NCs showed pH-dependent drug release behavior. Drug release from TiO2@ZnO–GO in neutral pH were higher than in acidic medium, due to anionic charge of GO nanosheet. MTT assay results showed that the curcumin-loaded NCs showed significant toxicity due to which cell viability reduced to below 50% at 140 μg/mL concentration, thereby confirming its anticancer effects. The goal of this study is the application of water-dispersed TiO2@ZnO–GO with pH-dependent release properties for design a new drug delivery carrier.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2016, over one and half million new cancer cases are diagnosed, and about six hundred thousand Americans were died of cancer the same year. Of these new cases in 2016, it is anticipated that one hundred thousands were be due to colon cancer. Indeed, colorectal cancer is the third leading reason of cancer death for both men and women [1]. However surgical removal of a primary colorectal tumor has proven to be beneficial, thirty percent of patients eventually develop a metastasis [2]. The most prevalent sites of metastasis are the liver, lungs, and draining lymph nodes [3]. Chemotherapy is one of the cancer therapy manners and many drugs such as irinotecan (CPT-11), doxorubicin (DOX), oxaliplatin (OXA), cisplatin (CP), and curcumin (CUR) have been used for chemotherapy [4,5,6].
CUR is the yellowish pigmentation of turmeric which is commonly used as a food flavoring and coloring agent. Its chemical formula is 1, 7-bis (4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3,5-dione with a chemical structure in the keto-enol tautomerism [7, 8]. CUR is an attracting therapeutic agent from a pharmaceutical viewpoint because of its notable biological characteristics, including its antioxidant, antimicrobial, anti-inflammatory, and wound healing activities [9, 10]. It also displays potential use for the medicinal treatment of various diseases, especially cancer [11]. However, CUR suffers from some disadvantages including low water solubility under acidic or neutral conditions, high decomposition rate in an alkaline media, and photo-degradation in organic solvents which subsequently limit its clinical applications [12]. The most challenging work in the progress of colon-specific drug delivery carriers is to deal with premature drug release from the carriers before the drug can be delivered to the target. To extend the successful colon-specific drug delivery NCs, they required to be stable and protected from the harsh acidic environment of the stomach and release the drug more specifically to the colon [13, 14]. Multiple approaches have been undertaken in recent years to develop the delivery of CUR in colonic tissues. Mucoadhesive chitosan nanoparticles [15], pH-sensitive nanoparticles [16], guar gum microspheres [17], polymeric NCs, [18] and graphene [19] have improved the bioavailability of CUR to some extent in colon, even so; these carriers did not deliver the soluble form of CUR.
Graphene (G), a two-dimensional single layer of carbon atoms in a closely packed honeycomb lattice, has attracted significant attention from scientific communities in recent years [20,21,22]. Graphene has been extensively studied in the last several years due to its interesting characteristics, such as significant electrical conductivity, great theoretical specific surface area and high chemical stability, excellent absorptivity, and excellent mechanical properties [23,24,25]. Graphene oxide (GO) is a derivative of graphene and has attracted attention of many bioapplications including cellular growth and differentiation [26], gene and drug delivery [27], and photo-thermal therapy [28]. GO can simultaneously activate toll-like receptor (TLR)-4 and -9 responses as same autophagy in macrophages [26] and colon cancer cell CT26. In addition, injection of GO alone stimulates the immune cell infiltration into the tumor bed and inhibits colon cancer growth in mice [29]. Furthermore, GO conjugated with chemotherapy drugs (DOX and CP-11) can improve the killing of MCF-7 cells that are resistant to DOX and CP [27].
Many research works are going on for the development of anticancer agents having enhanced targeted characteristics, better therapeutics and low toxicity [30,31,32,33]. Due to this, TiO2@ZnO are regarded to be a promising substance because of its reported anticancer potential [34, 35]. TiO2 is one of the well-known mesoporous materials. Because of biocompatibility, large surface area, and mesoporous properties of TiO2, some of the investigation has been developed to use nanosized TiO2 in dye-sensitized solar cells, electrochromic display, water splitting, degradation of organics, glucose sensors and specially as carrier for drug delivery which required slow and sustained release [36,37,38]. Biocompatibility and nontoxicity of TiO2 caused by combination of TiO2 with other materials such as mesoporous zinc oxide (mZnO) produce an intelligent drug delivery system for use in pharmaceutical formulation. In this work, we report the synthesis of a mesoporous TiO2@ZnO and TiO2@ZnO–GO by a facile sono-chemical way to allow multifunctional properties to be imparted into such a nanostructure. CUR was loaded on NCs, and the loading capacity and release behavior of CUR from NCs were also tested. As expected, the prepared (TiO2@ZnO–GO) have displayed high anticancer drug loading and pH-sensitive release behavior.
Materials and methods
Material
Zinc (II) nitrate hexahydrate (Zn (NO3)2·6H2O, ≥ 99%, M w = 290.70 g/moL), CUR (Merck, Darmstadt, Germany, Art No. 820354), sodium hydroxide (NaOH, M w = 40 g/moL), ethanol, and polyvinylpyrrolidone (PVP) surfactants were purchased from Merck. Deionized water was prepared by an ultra-pure water system (Smart-2-Pure, TKACo, Germany).GO was prepared from graphite powder using sulfuric acid (H2SO4, 98%), nitric acid (HNO3, 98%), potassium permanganate (KMnO4), 5% HCl, and hydrogen peroxide (H2O2, 30%), all purchased from Merck. Other solvents and chemicals were from chemical laboratory in purity grades.
Preparation of GO nanosheets
GO was synthesized from natural graphite powder by a modified Hummers method [35]. In a routine process, 1 g of graphite was added to 100 mL of concentrated H2SO4, 50 mL of HNO3 in an ice bath, and while stirring, 4 g of KMnO4 was slowly added. The reaction mixture is stirred for 2 h at temperatures below 10 °C, followed by 1 h at 35 °C until a thick dough had formed. After that, it is diluted by 100 mL of deionized (DI) water in an ice bath and transferred to a 98 °C oil bath and stirred for 1 h. Again, the mixture was diluted to 300 mL. Then, 20 mL H2O2 (30%) was added until the color of the solution changed to a light yellow. The final product was centrifuged and washed several times with 5% HCl solution, and then by DI water, until the supernatant became neutral. Finally, the final solid was dried at 60 °C for 24 h, and a loose brown powder was obtained.
Synthesis of TiO2@ZnO–1 wt% GO and TiO2@ZnO
At first, the TiO2 nanoparticles were synthesized by the sol–gel method [39]. A mesoporous ZnO shell was coated on the TiO2 nanoparticles using sono-chemical method. In a typical procedure, 0.11 g of the TiO2 nanoparticles was dispersed in a mixed solvent of ethanol: DI (1:1), 0.21 g PVP, and NaOH solution, followed by that, 1.8 g Zn (NO3)2·6H2O was added and exposed with ultrasound waves at room temperature for 5 h. Power and frequency of the ultrasound waves were 100 W and 20 kHz, respectively. Then, a suspension of 1 wt% of GO (prepared in mixture of 10 mL of DI and 20 mL of ethanol) was added to this solution at room temperature and stirred with magnetic stirring. The obtained ternary nanoparticles of TiO2@ZnO–GO were dried at 100 °C overnight. For comparison, the same method was used to synthesize TiO2@ZnO (m was short for mesoporous) with core–shell structure without GO.
Preparation of curcumin-loaded nanocarriers
CUR-loaded NCs were obtained by precipitation method by acetone as the solvent. Briefly, 15 mg of CUR was dissolved in 5 mL of acetone. Then, this solution was injected drop-wise into 50 mL of DI solution containing 60 mg of NCs under constant mixing rates and stirred magnetically at room temperature until complete evaporation of the acetone occurred and drug loaded nanoparticles were prepared. The final nanoparticles were separated by centrifuging at 20000 rpm for 15 min. The obtained NC was dried at vacuum oven overnight.
Characterization
XRD analysis
Phase analyses of the NCs were carried out by powder XRD (Philips X’ pert Pro MPD, Holland) using a graphite-filtered Cu Kα (k = 0.9 nm) radiation in the 2θ range of 10–90, at 2θ steps of 0.05, and a wavelength of 1.54 Å.
Particle morphology
The surface morphological properties of NCs were examined by means of scanning electron microscopy (SEM) (VEGA\\TESCAN-XMU) at an accelerating voltage of 20 kV, and transmission electron microscopy (TEM) images were obtained using a Philips CM120 microscope operating at 120 kV and linear resolution of 2.5 Å.
FT-IR analysis
FT-IR spectrophotometer (Bruker, Tensor 27, Biotage, Germany) was used for determining chemical structure of the NCs.
Determination of surface charge
The zeta potential of the prepared NCs was determined by dynamic light scattering (DLS) using a zetasizer (Malvern Instruments, Worcestershire, UK, model Nano ZS).
BET analysis
Nitrogen adsorption/desorption isotherms of TiO2@ZnO were measured by a BELSORP-mini II adsorption porosimeter at 77 K after being degassed at 180 °C under vacuum for 5 h. The specific surface areas were calculated using the Brunauer–Emmett–Teller (BET) method, and the pore size distributions were calculated according to the Barrett–Joyner–Halenda (BJH) method.
Determination of loading efficiency
To determine the loading efficiency of the drug in the NCs, two parameters were determined including the drug loading ratio of drug. Drug loading ratio was determined as:
where DL% is the drug loading ratio (percent), and W drug and W carrier represent the weight of the entrapped drug and the total weight of the corresponding drug-entrapped carrier, respectively.
For determination of the drug loading ratio, 1 mg of the final dried NCs was dissolved in 1 mL of acetone, and the drug content was measured spectrophotometrically (Thermo Fisher Scientific, USA, Madison, model GENESYS™ 10S) at wavelengths of 420 nm.
Drug release study
In general, drug-loaded NCs are suspended in a vessel containing certain amount of release media, and then the drug release is investigated over time. Briefly, 5 mg of drug-loaded carriers were dispersed in 20 mL phosphate-buffered saline (PBS) containing 1% (v/v) Tween 80, and the resulting suspension incubated at 37 °C. The tubes were centrifuged at 12000 rpm for 5 min to separate the particles from medium, Then, at predetermined time intervals, 1 mL of the suspension was taken out and replaced by 1 mL fresh PBS. The concentration of CUR in the suspension was determined by ultraviolet–visible spectroscopy (UV–Vis) at wavelength of 420 nm. All the release studies were carried out in triplicate. In order to study the pH-dependency of the drug release, the experiments were carried out as well as at a pH of 5.5.
Cell cytotoxicity
For the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay, 96 well plates were used, and Caco-2 cells were seeded onto plates and adhered and grown overnight in 200 μL culture medium with 104 cancer cells per well concentration. Then, the cancer cells were incubated with 100 μL fresh medium containing serial concentrations (0–140 μg/mL) of void CUR and same doses of CUR/TiO2@ZnO–GO and CUR/TiO2@ZnO for 24 h and a group without treatment for control. Then 20 μL of 4 mg/mL MTT solution was added to each 100 μL media of well and the plates were then incubated at 37 °C in a humidified atmosphere containing 95% air and 5% CO2 for 3 h. Following the incubation, the MTT solution was eliminated, and 100 μL of dimethyl sulfoxide (DMSO) was added to each well for dissolution of the formazan crystals. The plates were shaken for 10 min on a plate shaker to obtain adequate solubility. After the shaking, absorbance readings of each well were done at 570 nm (single wavelength) by a multi-scan plate reader made in the UK. All experiments were repeated three times for statistical analysis. The final results were expressed as mean ± S.D.
Results and discussion
XRD
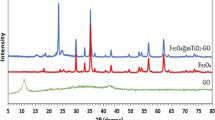
The Bragg diffraction peaks of the two NCs, shown in Fig. 1, agreed well with those of the pure synthesized hexagonal ZnO [40], at 2θ values of 31.9°, 34.6°, 36.4°, 47.7°, 56.8°, 63.0°, and 68.1° which were indexed to be (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), and (1 1 2) planes, respectively. and the peaks of 2θ = 25.6°, 30.1°, 38.0°, 48.7°, and 54.1° can be indexed to the crystal faces of (1 0 1), (1 2 1), (0 0 4), (2 0 0), and (1 0 5) of TiO2, respectively. Due to the low amount of graphene oxide in the composite, the (0 0 2) peak of the corresponding to an interlayer distance of GO nanosheets around 21° has slight intensity and shown in the inset Fig. 1 [41].
Particle morphology
Figure 2a, b shows the SEM image of TiO2@ZnO–GO (a) and TiO2@ZnO (b). TiO2 nano particles are used as a template to produce fine ZnO powders. Last researches indicate that suitable amount of TiO2 can effectively suppress grain growth and phase transformation of ZnO [42]. With this approach, we can produce uniform nanoparticles. It can be seen in Fig. 2a that the graphene nanosheets are fully exfoliated and almost decorated with nearly spherical TiO2@ZnO nanoparticles. Figure 2b illustrates the SEM images of TiO2@ZnO nanoparticles. It is evident from the Fig. 2b that the core–shell nanoparticles have a clear hexagonal phase with an average size of 190 nm. Figure 3 shows TEM images of TiO2@ZnO core–shell nanoparticles, which show the agglomeration of particles with different shapes (both spherical and hexagonal shape can be observed). From TEM images, the average particle size was estimated to be around 190 nm for nanoparticles, which is consistent with the SEM results.
FT-IR analysis
FT-IR spectra of the NCs are shown in Fig. 4. Nevertheless, the significant bands that characterize the formation of TiO2@ZnO are located at 470 and 657 Wavenumber (cm−1), which are associated with the vibrations of Zn–O and Ti–O bonds, respectively [43, 44]. The peaks at 1085, 1530 cm−1 were attributed to the stretching vibrations of C–O and C–H bonds, respectively [45]. In the case of the GO nanosheets, the absorption peak appeared at 3440 cm−1 in the IR spectra. This confirmed the presence of –OH groups in its nanostructure.
Surface charge of nanocarrier
The zeta potential of the aqueous suspension of NCs was also measured. Figure 5 shows zeta potential for TiO2@ZnO–GO determined in the PBS buffer solution at pH 7.4 and 5.5, respectively. As shown in Fig. 5, zeta potential of CUR-loaded NCs at pH 7.4 and 5.5 was found to be about − 15.8 and − 0.31 mv. It is clear that at the pH 7.4 where our results showed a negative zeta potential due to carboxyl group of graphene oxide are ionized. At lower pH, the carboxyl group of graphene oxide are in deionized form which could form a proper condition for π–π stacking bond between drug and nanocarrier [46]. Surface charge is important in decisive whether the nanoparticles will cluster in blood flow or will stick to or interact with oppositely charged cell membrane. The plasma and blood cells constantly had a negative charge; nanoparticles with slight negative surface charge can minimize nonspecific contact with these components through electrostatic interactions [33, 47].
BET analysis
The nitrogen adsorption/desorption isotherms and pore size distribution profiles of TiO2@ZnO are presented in Fig. 6. It can be seen, sample have microporous structure. The BET surface area and total pore volume of TiO2@ZnO were calculated to be 31.59 m2/g and 0.084 cm3/g, respectively. As we knew, the surface areas and pore volumes increased systematically with utilizing TiO2. Similar trends were observed after loading or mixing TiO2 with ZnO [48]. Researches showed that surface area of pure ZnO is about 11 m2/g [49], so with this approach, surface area would be significantly higher than pure ZnO.
Drug loading and release study
The effect of TiO2@ZnO and TiO2@ZnO–GO on the loading and release of CUR were investigated in detail in this section. Figures 7 and 8 show a schematic diagram of CUR loading and release for NCs. The amounts of CUR incorporated as pure drug in these NCs are listed in Table 1. It is observed that the loading of CUR in the NCs increases when utilizing TiO2@ZnO–GO instead of TiO2@ZnO. This could be attributed to the strong π–π stacking interaction between GO and drugs, which allows the penetration of drug molecules into the NCs [46]. Figure 9 illustrates in vitro drug release profile of CUR-loaded in NCs at PH 5.5 and 7.4. The results revealed the bare curcumin release percents attainable after a period of 12 h in PBS pH 7.4 and 5.5 were 86 and 88%, respectively. As expected, percentage of CUR released from the NCs slightly increased as the pH value increased from 5.5 to 7.4. The drug release curve Fig. 9 clearly illustrates that at the 12-h, there is a 19 and 28% CUR release from TiO2@ZnO to GO matrix which reached at 29s and 49% at pH 5.5 and 7.4, respectively, after six days. Sustained release of curcumin can be attributed to the entrapment of curcumin in mesoporous of NCs. The release is faster in neutral pH than acidic pH which can be attributed to deprotonation of residual carboxyl groups in GO of the TiO2@ZnO–GO at higher pH, creating a repulsion force between the NC and the drug. Therefore, more amount of drug will be diffused out. In addition in neutral medium, the breaking of hydrogen bonding between the GO and CUR facilitated the release of the drug and thus increased the cumulative release. As expected, considerable initial burst curcumin release was observed from the NCs due to desorbing curcumin from surface of NCs. This phenomenon could be useful for delivering drug to colon because after about 6 h the NCs reach to colon, therefore significant drug release should be happen during this period of time. In other hand, CUR released from NCs that could expose ZnO to cancer cells and bare ZnO makes this synergistic effect. These results reveal that TiO2@ZnO–GO could be used as colon-specific drug delivery carriers that would show pH-dependent drug release behavior [50].
Study on cancerous cell’s viability after CUR loaded TiO2@ZnO and TiO2@ZnO–GO NCs treatment
The in vitro anticancer effects of CUR-loaded NCs against human epithelial colorectal adenocarcinoma cells (Caco-2) (pH 6.2–6.9) estimated using a MTT assay (Fig. 10). MTT results illustrated that the Curcumin-loaded NCs showed significant toxicity due to which cell viability reduced to below 50% at 140 μg/mL concentration, thereby confirming its anticancer effect. The data exhibit that cell toxicity is directly proportionate to CUR-loaded NCs concentration. The concentration at which cell growth was inhibited by 50% (IC50) was specified by the standard curve method and is shown in Table 2. Higher toxicity of TiO2@ZnO and TiO2@ZnO–GO than CUR-loaded NCs can be imputed to the presence of ZnO nanoprecipitation on the cells. In particular, ZnO when reduced from bulk to nanoscale has been shown to exhibit inherent preferential cytotoxicity against cancer cells in vitro [51, 52]. Also, the cytotoxicity assay showed relatively higher toxicity of the TiO2@ZnO than TiO2@ZnO–GO against Caco-2 cancer cell lines. Therefore, GO reduced toxicity of ZnO nanoparticle. On other hand, curcumin-loaded NCs showed lower toxicity in the test revealed that adsorbed curcumin on surface of ZnO nanoparticles covered their surface and this method can reduce toxicity of ZnO nanoparticles. And due to lower release of drugs in acidic environment like stomach, this method can protect normal cells of stomach against toxicity of ZnO. In other hand, when the physiological pH was increased (at colon), CUR released from NCs and bare ZnO makes this synergistic effect. Also, the comparable anticancer activity of NCs and curcumin revealed that curcumin retained its anticancer activity even after being loaded into NCs.
Conclusion
Novel TiO2@ZnO–GO NC was synthesized as a pH-sensitive carrier for colon-targeted drug delivery. The SEM and TEM studies revealed that the prepared NCs had a diameter about 190 nm and hexagonal in shape. The TiO2@ZnO–GO showed pH-dependent drug release behavior due to the presence of large amount of carboxylic acid group in the GO network. The release of CUR from the TiO2@ZnO–GO was dependent on pH of the medium. It was found that the rate and amount of CUR released from the TiO2@ZnO–GO are higher in pH 7.4. MTT results showed that the curcumin-loaded NCs showed significant toxicity due to which cell viability reduced to below 50% at 140 μg/mL concentration, thereby confirming its anticancer effects. These results demonstrate that TiO2@ZnO–GO could be a drug delivery system to deliver the drug more specifically to the colon.
References
Yancik R, Ries LA (2000) Aging and cancer in America: demographic and epidemiologic perspectives. Hematol Oncol Clin North Am 14(1):17–23
Schlüter K et al (2006) Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol 169(3):1064–1073
Ko F-C et al (1999) Risk and patterns of brain metastases in colorectal cancer. Dis Colon Rectum 42(11):1467–1471
Tournigand C et al (2012) Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 30:3353–3360
Danafar H et al (2017) Co-delivery of hydrophilic and hydrophobic drugs by micelles: a new approach using drug conjugated PEG–PCLNanoparticles. Drug Dev Ind Pharm 43(11):1908–1918
Danafar H et al (2017) Investigation of drug delivery of rattle-structured gold nanorod-mesoporous silica nanoparticles core–shell as curcumin carrier and their effect on MCF7 and 4T1 cell lines. J Zanjan Univ Med Sci Health Serv 25(113):124–136
Kheiri Manjili H et al (2017) Pharmacokinetics and in vivo delivery of curcumin by copolymeric mPEG-PCL micelles. Eur J Pharm Biopharm 116:17–30
Danafar H (2017) Study of the composition of polycaprolactone/poly (ethylene glycol)/polycaprolactone copolymer and drug-to-polymer ratio on drug loading efficiency of curcumin to nanoparticles. Jundishapur J Nat Pharm Prod 12(1):e34179
Sun Y-M et al (2002) Theoretical elucidation on the antioxidant mechanism of curcumin: a DFT study. Org Lett 4(17):2909–2911
Panchatcharam M et al (2006) Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem 290(1–2):87–96
Sun A et al (2006) Synthesis of EF24-tripeptide chloromethyl ketone: a novel curcumin-related anticancer drug delivery system. J Med Chem 49(11):3153–3158
Tønnesen HH, Karlsen J, van Henegouwen GB (1986) Studies on curcumin and curcuminoids VIII. Photochemical stability of curcumin. Z Lebensm Unters Forsch 183(2):116–122
Tu H et al (2010) Study of the sigmoidal swelling kinetics of carboxymethylchitosan-g-poly (acrylic acid) hydrogels intended for colon-specific drug delivery. Carbohyd Polym 82(2):440–445
Rao KM et al (2014) pH sensitive halloysite-sodium hyaluronate/poly (hydroxyethyl methacrylate) nanocomposites for colon cancer drug delivery. Appl Clay Sci 97:33–42
Chuah LH et al (2014) Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf B 116:228–236
Zhang L et al (2011) Eudragit® S100 coated calcium pectinate microspheres of curcumin for colon targeting. J Microencapsul 28(7):659–667
Madan J et al (2014) Cyclodextrin complexes of reduced bromonoscapine in guar gum microspheres enhance colonic drug delivery. Mol Pharm 11(12):4339–4349
Dutta RK, Sahu S (2012) Development of a novel probe sonication assisted enhanced loading of 5-FU in SPION encapsulated pectin nanocarriers for magnetic targeted drug delivery system. Eur J Pharm Biopharm 82(1):58–65
Chen G-Y et al (2015) Graphene oxide as a chemosensitizer: diverted autophagic flux, enhanced nuclear import, elevated necrosis and improved antitumor effects. Biomaterials 40:12–22
Novoselov K et al (2005) Two-dimensional atomic crystals. Proc Natl Acad Sci USA 102(30):10451–10453
Calizo I et al (2007) Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett 7(9):2645–2649
Liu L et al (2008) Graphene oxidation: thickness-dependent etching and strong chemical doping. Nano Lett 8(7):1965–1970
Huang JC (2002) Carbon black filled conducting polymers and polymer blends. Adv Polym Technol 21(4):299–313
Ishigami M et al (2007) Atomic structure of graphene on SiO2. Nano Lett 7(6):1643–1648
Yan J et al (2007) Raman scattering and tunable electron–phonon coupling in single layer graphene. Solid State Commun 143(1):39–43
Chen G-Y et al (2012) A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 33(2):418–427
Zhang L et al (2010) Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 6(4):537–544
Tao Y et al (2014) Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials 35(37):9963–9971
Chen GY et al (2014) Graphene oxide triggers toll-like receptors/autophagy responses in vitro and inhibits tumor growth in vivo. Adv Healthc Mater 3(9):1486–1495
Danafar H et al (2017) Polylactide/poly(ethylene glycol)/polylactide triblock copolymer micelles as carrier for delivery of hydrophilic and hydrophobic drugs: a comparison study. J Pharm Investig. doi:10.1007/s40005-017-0334-8
Nosrati H et al (2017) Green and one-pot surface coating of iron oxide magnetic nanoparticles (IONPs) with natural amino acids and its biocompatibility investigation. Appl Organomet Chem. doi:10.1002/aoc.4069
Gharebaghi F et al (2017) Preparation of wormlike polymeric nanoparticles coated with silica for delivery of methotrexate and evaluation of anticancer activity against MCF7 cells. J Biomater Appl 31(9):1305–1316
Nosrati H et al (2017) New advances strategies for surface functionalization of iron oxide magnetic nano particles (IONPs). Res Chem Intermed. doi:10.1007/s11164-017-3084-3
Rasmussen JW et al (2010) Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv 7(9):1063–1077
Rahimi-Nasrabadi M et al (2016) Synthesis and characterization of ZnFe2−xYbxO4–graphene nanocomposites by sol–gel method. J Mater Sci Mater Electron 27(11):11940–11945
Vallet-Regí M, Balas F, Arcos D (2007) Mesoporous materials for drug delivery. Angew Chem Int Ed 46(40):7548–7558
Lopez T et al (2009) Kinetic study of controlled release of VPA and DPH antiepileptic drugs using biocompatible nanostructured sol–gel TiO2. J Mater Sci 44(20):5459–5468. doi:10.1007/s10853-009-3745-8
Hamadanian M, Rostami M, Jabbari V (2017) Graphene-supported C–N–S tridoped TiO2 photo-catalyst with improved band gap and charge transfer properties. J Mater Sci Mater Electron 28(20):15637–15646
Rostami M et al (2017) Facile synthesis and characterization of TiO2–graphene–ZnFe2−xTbxO4 ternary nano-hybrids. J Mater Sci. doi:10.1007/s10853-017-0934-8
Sun H et al (2014) A comparative study of reduced graphene oxide modified TiO2, ZnO and Ta2O5 in visible light photocatalytic/photochemical oxidation of methylene blue. Appl Catal B 146:162–168
Li M et al (2013) In situ synthesis and biocompatibility of nano hydroxyapatite on pristine and chitosan functionalized graphene oxide. J Mater Chem B 1(4):475–484
Asl SK, Rad MK, Sadrnezhaad S (2011) Hydrothermal preparation of TiO2–ZnO nano core‐shell structure with quantum size effect. In: AIP conference proceedings, vol 1400. doi:10.1063/1.3663156
Ganesh M et al (2012) Drug release evaluation of mesoporous TiO2: a nano carrier for duloxetine. In: Computer applications for modeling, simulation, and automobile, vol 341. Springer, pp 237–243
Anchieta CG et al (2014) Effects of solvent diols on the synthesis of ZnFe2O4 particles and their use as heterogeneous photo-Fenton catalysts. Materials 7(9):6281–6290
Song M, Xu J (2013) Preparation of polyethylenimine-functionalized graphene oxide composite and its application in electrochemical ammonia sensors. Electroanalysis 25(2):523–530
Upadhyaya L et al (2015) Efficient water soluble nanostructured ZnO grafted O-carboxymethyl chitosan/curcumin-nanocomposite for cancer therapy. Process Biochem 50(4):678–688
Danafar H et al (2017) Drug-conjugated PLA–PEG–PLA copolymers: a novel approach for controlled delivery of hydrophilic drugs by micelle formation. Pharm Dev Technol 22(8):947–957
Al-Johani MS, Al-Zaghayer Y, Al-Mayman SI (2015) TiO2/ZnO photocatalytic activity for hydrogen production. Int Sci J Environ Sci 4:1–8
Liang Y et al (2015) Preparation of porous 3D Ce-doped ZnO microflowers with enhanced photocatalytic performance. RSC Adv 5(74):59887–59894
Seeli DS et al (2016) Guar gum succinate-sodium alginate beads as a pH-sensitive carrier for colon-specific drug delivery. Int J Biol Macromol 91:45–50
Hanley C et al (2008) Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 19(29):295103
Wang H et al (2009) Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. J Mater Sci Mater Med 20(1):11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zamani, M., Rostami, M., Aghajanzadeh, M. et al. Mesoporous titanium dioxide@ zinc oxide–graphene oxide nanocarriers for colon-specific drug delivery. J Mater Sci 53, 1634–1645 (2018). https://doi.org/10.1007/s10853-017-1673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1673-6