Abstract
A flexible and stretchable three-dimensional carbon electrode (SCF) based on the natural woven fabric was developed by coupling a sublimation generating pores method and facile pyrolyzing procedure. The SCF electrode possessed good mechanical flexibility, which was attributed to the increase in the specific surface area and the amount of the pores. Furthermore, the SCF electrode exhibited excellent capacitance property (157.89 F g−1 at 2 m s−1), resulting from the synergistic effect between the double-layer capacitance (large specific surface area, high porosity and excellent conductivity) and the pseudocapacitance of O and N heteroatoms. The SCF will be employed as a promising candidate for flexible energy storage devices owing to the excellent wearable and electrochemical performance. The one-step strategy can provide a novel route for low-cost production of high-performance flexible energy storage materials from the natural fabrics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, a rapidly increasing research progress has been made in wearable electronics owing to its remarkable applications in micro-robotics, energy harvesting, military equipment for soldiers, electronic textiles and implantable medical devices. As an indispensable part in a wearable system, a high flexible energy storage device with lightweight and high performance represents a promising material to power the functioning devices [1,2,3]. Among the diverse types of flexible substrates, fabric-based materials made by pressing or weaving natural or synthetic fibers are employed as candidates for energy storage devices because of their soft, porous nature and three-dimensional (3D) structure [4, 5]. In general, the natural fabrics are nonelectric conductivity whose specific resistance is more than 106–107 Ω sq−1. It is crucial for the fabric-based energy storage to have good electrical conductivity. Up to now, there are two strategies to convert the common fabrics into the conductive 3D frameworks. One method is coating the pretreated cotton or polyester fabrics, with carbon materials or electrically conducting polymers [6, 7]. Cui et al. [8]. developed a dyeing-like approach to obtain the conductive fabrics by dipping single-walled carbon nanotubes (SWCNTs) onto cotton fabrics. The reported material possessed highly conductive and the specific capacitance reached 70–80 F g−1 at 0.1 A g−1. However, in this method, the using of SWCNTs increased the cost of the devices, which hampered the scale-up application of this material. Compared with the coated fabric, the other method is directly converting the traditional fabrics into carbonized fabrics (CF) by the thermal treatment to generate the conductive textured carbons [9, 10]. The direct conversion carbonized fabrics not only expressed high electrical conductivity but also maintained the compatibility with textiles, which represented a type of lightweight flexible materials. However, when the fibers were being carbonized to produce carbon fibers under a high temperature, the formed CF easily suffered from a low porosity and the CF exhibits quite brittle. In addition, the lower capacitance becomes another drawback for the directly carbonized fabrics, even though several researches have been done to improve their properties. For example, Bao et al. [9]. fabricated the activated carbon textile (ACF) by directly carbonizing the cotton T-shirt using the NaF solution, which showed the specific capacitance of 70.2 F g−1 at a scan rate of 2 mV s−1 in 1 M Na2SO4 aqueous solution. Chen et al. [10] carbonized the flax textile to obtain the carbon fiber cloth, which exhibited a low specific capacitance of 0.78 F g−1 at 0.1 A g−1 in 0.1 M Na2SO4 electrolyte. Therefore, developing the excellent electrochemical performance and flexible mechanical carbon electrodes has been one of the greatest challenges associated with the wearable electronics.
Creating porosity is demonstrated to be an effective approach to improve the flexible properties of materials [11, 12]. The main reason is that the pores play an important role to reduce the stresses, resulting in the flexibility improvement of the materials, when the materials are bent. Some research groups reported several pore generating methods based on the preparation of carbon nanofibers, such as utilizing pore generating agents or post-activation methods [13,14,15,16]. However, none of the above methods can change the porosity of fabrics to enhance the mechanical properties, because the generating pores agents in these methods formidably create the pores in the cellulose fibers due to their high crystallinity. Song et al. [17] developed a sublimation method using terephthalic acid (PTA) as the sublimating agent, which effectively improved the flexibility of the electrospun carbon nanofiber film. Cotton, an abundant, low-cost and environmental friendliness natural source, has a complex hierarchical structure, such as the crystalline and amorphous regions, microfibrillar structure, cavities, the pores and the spaces between the microfibrils. These structures are called as the accessibility of fibers, which are more receptive to chemical agents and directly affect the absorption amount of chemical molecules in the fibers. PTA as a light acid with small molecules can be uniformly dispersed in DMF and fully absorbed inside the 3D network of the fabrics. During the heat treatment, PTA absorbed inside the cellulose fibers matrix easily sublimed and large amount pores were generated, which could not only increase the BET and pore volume, but also enhance the flexibility. Furthermore, oxygen and nitrogen atoms can be successfully introduced in the carbonized fabrics by the sublimation procedure using PTA and DMF as the solvent, which is beneficial to the improvement of the electrochemical properties.
Based on the above considerations, we employed a simple and convenient strategy to realize a cheap flexible carbonized fabric electrode (called as SCF) with high energy storage performance by coupling a sublimation generating pores method and facile thermal treatment. The mechanical flexibility of SCF was improved by a sublimation approach to produce high porosity with micropores and mesopores inside the fabric and large BET (Table S1). The capacitance performance was largely enhanced with respect to the multiple synergistic effects, such as a large specific surface area, high volume of hierarchical pores, oxygen and nitrogen enrichment and excellent inter-connected electrical conductive frameworks.
Experimental
Synthesis of flexible carbonized fabric electrode
The natural cotton fabric (228 g cm−2) was impregnated with 15 g L−1 NaOH at a temperature 95 °C for 1 h to remove the impurities (waxes, protein and other impurities), which can effectively improve fiber absorption capacity. The pretreated fabric was dipped in 10 g DMF containing 0.5 g PTA [17], dried at room temperature and then pyrolyzed at 800 °C for 1 h with a heating rate of 2 °C/min in an argon atmosphere. Finally, the autoclave was allowed to cool to room temperature. The PTA absorption amount in DMF solvent depended on the surface properties of the pretreated cotton fabrics.
Characterization
The surface morphology and microstructure were assessed by scanning electron microscopy (HITACHI/TM-1000, Japan, at 15 kV) and transmission electron microscopy (JEM-2100F, Japan). The crystallographic structure was determined by powder X-ray diffraction (with the 2θ from 0 to 90o) on a D/max 2550 VB/PC equipped with Cu Kα radiation. Raman spectra were conducted on inVia-Reflex with an excitation wave length of 633 nm. Nitrogen adsorption–desorption isotherms were performed on TriStar II 3020 M at 77 K. X-ray photoelectron spectroscopy was collected on an XPS-700 spectrometer.
Electrochemical measurement
All the electrochemical performances were tested in 1 M Na2SO4 electrolyte at room temperature using CHI 660E electrochemical workstation (Shanghai Chenhua Instruments Co.). In the three-electrode system, the SCF with an effective area of 1 cm2, a Pt plate and a Ag/AgCl were used as working, counter and reference electrode, respectively. Cyclic voltammetry, galvanostatic charge/discharge and electrochemical impedance spectroscopy in the frequency range from 100 to 0.01 Hz with the amplitude of 5 mV were used to evaluate the electrochemical characterization. The specific capacitance, C (F g−1), was calculated from the cyclic voltammetry curves by using the following equation:
where s is the scan rate (V s−1), m is the mass of the active material (g), ∆V is the voltage change (V).
Results and discussion
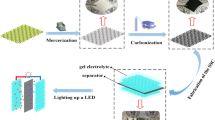
The preparation process of SCF is illustrated in Scheme 1. The white cotton fabric with the thickness of 0.32 mm was converted into the black carbonized fabric with the thickness of 0.25 mm. Meanwhile, the diameter of the fiber was reduced to 7–9 μm (as shown in Fig. (d) of Scheme 1), which was attributed to the thermal decomposition of some organic components in the fibers. From digital photographs and SEM images of SCF (Fig.c, d and e of Scheme 1), we can see the morphology of the hierarchical fabric after the carbonization process still maintained original weave structures with interlacing warp and weft yarns in the vertical and horizontal directions. The fiber also presented a flat ribbon character with hollow structure, which was similar with the natural cotton fiber. (Fig. S1). High-resolution transmission electron microscopy (HRTEM) images further confirmed the hollow porous structure and the amorphous graphite nature of the carbonized fiber as Fig. 1. The prepared SCF also expressed lightweight and flexible mechanical so that it could be stretchable, folded and rolled into the multilayer structures as demonstrated in Scheme 1. Furthermore, there is no obvious change in the conductivity of SCF under stretched and rolled conditions, which can be seen from Table S2.
From TEM images of Fig. 1, we can see a qualitative indication of increased porosity due to the sublimation and carbonization procedures. The surface area and pore structures of SCF were tested by N2 adsorption–desorption measurement, and the results are shown in Fig. 1d. The nitrogen adsorption isotherm of the SCF demonstrated the Type IV behavior indicative of a large amount of micropores contribution and the presence of mesopores. The pore size distribution curve represented the prepared SCF has a hierarchical pore structure, including micropores with below 2 nm diameter and mesopores with 3.9 nm diameter (Fig. S2). It has been proved that the microporous nature of materials could be valuable for an enhanced capacitance on the double-layer capacitor applications and the existence of these mesopores would improve the surface area for ion transportation and enhance the electrochemical performance [18,19,20]. Meanwhile, the SCF possessed a larger specific surface area (BET) of 841.76 m2 g−1, compared to the CF BET of 432.54 m2 g−1 (Fig. S3) which was mainly attributed to the micropores and mesopores predominately generated by PTA sublimation.
The crystalline structure of the SCF was identified by XRD measurement, and the results are exhibited in Fig. 1e. The SCF displayed two relatively sharp diffraction peaks with 2θ located at 22.2° and 43.7°, corresponding to the (002) plane of amorphous carbon and (101) plane of the graphitic character of SCF, respectively. The diffraction peak indexed to the (002) plane is obviously sharper than that of 101 plane, which suggested that the SCF has more perfection of graphitic crystalline structure. These results are supported by the Raman spectra as shown in Fig. 1f. Raman spectra of SCF showed the characteristic D and G bands at 1337 and 1597 cm−1, respectively. The relative intensity ratio of the D and G bands ID/IG can be calculated as an indication of the molecular order within the crystalline carbon structure, which is related to the size of the graphite crystallite [21]. This proved a more order crystalline carbon structure in the SCF, which most probably results from introducing graphitization by PAT activation of the natural cotton at 800 °C. PTA can easily infiltrate into the cotton matrix, which causes a rearrangement of amorphous carbons under the appropriate thermodynamic condition (Fig. S4).
XPS was performed to explore the elemental states and the composition of SCF. The atom percentages of C, O and N of SCF were found to be 87.27, 11.08 and 1.64 at%. The C 1 s XPS spectra consisted of three peaks at 286.8, 287.3 and 288.7 eV pointing toward C = O, C–O and C–N group, respectively. The O 1 s spectra exhibited three different types of oxygen groups in SFC, which were located at 513.3 eV due to C=O group and at 532.5, 534.8 eV resulting from C–O, Ph-O-(C=O)-Ph, respectively. The increased N and O contents were most likely a result of the doping of PTA and solvent DMF in the sublimation step. Heteroatoms N and O enrichment into the carbon frameworks can enhance the conductivity (Table S3), pseudocapacitance and the wettability of carbon materials resulting in the enhancement of the specific capacitances [22]. The movie. S1 displays the hydrophilicity of SCF, which is important for carbon electrode material. The elemental mapping (C, O, N) of the SCF is observed in Fig. 2d, e and f, suggesting the uniform element distributions of N and O in the SCF by the dipping method.
The SCF possessed an excellent capacitive behavior as indicated by a quasi-rectangular shape in Fig. 3a. The specific capacitance of SCF electrode was calculated to be 157.89 F g−1 at the scan rate of 2 mV s−1, which was better than that of ACF (70.2 F g−1) prepared by solution-processed [9]. The galvanostatic charge/discharge curves of SCF at different current densities are depicted in Fig. 3b. The charge/discharge lines retained nearly triangular shapes over a wide range of current densities, demonstrating an ideal double-layer capacitor behavior. Compared with the ACF, the dramatic enhancement in capacitance of SCF can be partly attributed to the increased double-layer capacitance resulting from the large surface area and high porosity of the SCF. Some researches have proved that the micropores smaller than 2 nm greatly increase the double-layer charge storage, which promises facile ion diffusion and fast electron transport [23]. Nevertheless, it is impossible for SCF that the improved capacitance of SCF is originated from the only factor of the enhanced double-layer capacitance. The most likely source of a significant portion of this capacitance originates from the pseudocapacitive contributions of the heteroatoms [22, 24]. XPS and EDS results have confirmed the presence of nitrogen and oxygen atoms in the network structure and the surface of SCF. As shown in Fig. 3c, the SCF electrode has not only a relatively low equivalent series resistance but also a much shorter Warburg region, indicating high conductivity and fast ion transport, with a typical sheet resistance of 20–30 Ω sq−1. The increase in the electrical conductivity from SCF is in agreement with the XRD, Raman spectra and TEM results. The cycle stability of the SCF was measured under the scan rate of 100 mV s−1 for 2000 cycles and maintained about 90% capacitance retention after 2000 cycles in Fig. 3d. The excellent electrochemical performances of SCF further demonstrated the synergistic effect between the double-layer capacitance (large specific surface area, high porosity and excellent conductivity) and the pseudocapacitance of N and O heteroatoms.
Conclusions
In summary, a flexible and binder-free 3D porous carbon framework electrode was fabricated by the combination of the sublimation and the carbonizing method based on cotton woven fabrics for the first time. We employed PTA in DMF to restructure the natural cotton fabrics for obtaining hierarchical porous texture and high graphitization combined together, resulting in a good mechanical flexibility and conductivity. PTA as the sublimation agent can create additional pores, which can not only increase the specific surface area to improve the double capacitance, but also enhance the porosity of SCF to improve the flexibility. The carbonizing cotton fabric process generated the 3D carbon network with high content graphite, which enhanced the whole conductivity and retained the carbon material as the free-standing electrode. The 3D SCF exhibited excellent electrochemical performance and wearable as the flexible energy storage. The one-step strategy by the sublimation and carbonization can provide a novel route for low-cost production of high-performance energy storage materials with excellent flexible mechanical from the natural fabrics.
References
Beidaghi M, Gogotsi Y (2014) Capacitive energy storage in micro-scale devices: recent advances in design and fabrication of micro-supercapacitors. Energy Environ Sci 7(3):867
Liu LB, You Y, Yan C, Kan L, Zheng ZJ (2015) Wearable energy-dense and power-dense supercapacitor yarns enabled by scalable graphene–metallic textile composite electrodes. Nat Commun 6:7260
Bahk JH, Fang H, Yazawa K, Shakouri A (2015) Flexible thermoelectric materials and device optimization for wearable energy harvesting. J Mater Chem C 3(40):10362–10374
Jost K, Dion G, Gogotsi Y (2014) Textile energy storage in perspective. J Mater Chem A 2(28):10776–10787
Peng M, Cai X, Fu YP, Yu X, Liu SQ, Deng B, Hany K, Zou DC (2014) Facial synthesis of SnO2 nanoparticle film for efficient fiber-shaped dye-sensitized solar cells. J Power Sour 247(2):249–255
Anothumakkool B, Bhange SN, Soni R, Kurungot S (2015) Novel scalable synthesis of highly conducting and robust PEDOT paper for high performance flexible solid-supercapacitor. Energy Environ Sci 8(4):1339–1347
Li J, Cheng XQ, Shashurin A, Keidar M (2012) Review of electrochemical capacitors based on carbon nanotubes and graphene. Graphene 1(1):1–13
Pasta M, Mantia FL, Hu LB, Deshazer HD, Cui Y (2010) Aqueous supercapacitors on conductive cotton. Nano Res 3(6):452–458
Bao LH, Li XD (2012) Towards textile energy storage from cotton T-shirts. Adv Mater 24(24):3246–3252
He SJ, Chen W (2015) Application of biomass-derived flexible carbon cloth coated with MnO2 nanosheets in supercapacitors. J Power Sour 294(294):150–158
Zhang XQ, Sun Q, Dong W, Li D, Lu AH, Mu JQ, Li WC (2013) Synthesis of superior carbon nanofibers with large aspect ratio and tunable porosity for electrochemical energy storage. J Mater Chem A 1(33):9449–9455
Mccann JT, Li D, Xia YN (2005) Electrospinning of nanofibers with core-sheath, hollow, or porous structures. J Mater Chem 15(7):735–738
Niu HT, Zhang J, Xie ZL, Wang XG, Lin T (2011) Preparation, structure and supercapacitance of bonded carbon nanofiber electrode materials. Carbon 49(7):2380–2388
Kim C, Ngoc BTN, Yang KS, Kojima M, Kim YA, Kim YJ, Endo M, Yang SC (2007) Self-sustained thin webs consisting of porous carbon nanofibers for supercapacitors via the electrospinning of polyacrylonitrile solutions containing zinc chloride. Adv Mater 19(17):2341–2346
Yang Y, Centrone A, Chen L, Simeon F, Hatton TA, Rutledge GC (2011) Highly porous electrospun polyvinylidene fluoride (PVDF)-based carbon fiber. Carbon 49(11):3395–3403
Kim C, Jeong YI, Ngoc BTN, Yang KS, Kojima M, Kim YA, Endo M, Lee JW (2007) Synthesis and characterization of porous carbon nanofibers with hollow cores through the thermal treatment of electrospun copolymeric nanofiber webs. Small 3(1):91–95
Liu H, Cao CY, Wei FF, Huang PP, Sun YB, Jiang L, Song WG (2013) Flexible macroporous carbon nanofiber film with high oil adsorption capacity. J Mater Chem A 2(10):3557–3562
Fang Y, Lv YY, Che RC, Wu HY, Zhang XH, Gu D, Zheng GF, Zhao DY (2013) Two-dimensional mesoporous carbon nanosheets and their derived graphene nanosheets: synthesis and efficient lithium ion storage. J Am Chem Soc 135(4):1524–1530
Feng D, Lv YY, Wu ZX, Dou YQ, Han L, Sun ZK, Xia YY, Zheng GF, Zhao DY (2011) Free-standing mesoporous carbon thin films with highly ordered pore architectures for nanodevices. J Am Chem Soc 133(38):15148–15156
Yun YS, Cho SY, Shim J, Kim BH, Chang SJ, Baek SJ, Huh YS, Tak Y, Park YW, Park S (2013) Microporous carbon nanoplates from regenerated silk proteins for supercapacitors. Adv Mater 25(14):1993–1998
Dresselhaus MS, Dresselhaus G, Jorio A, Filho AGS, Saito R (2002) Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 40(12):2043–2061
Hao L, Li XL, Zhi LJ (2013) Carbonaceous electrode materials for supercapacitors. Adv Mater 25(28):3899–3904
Simon P, Gogotsi Y (2012) Capacitive energy storage in nanostructured carbon–electrolyte systems. Acc Chem Res 46(5):1094–1103
Zheng GY, Hu LB, Wu H, Xie X, Cui Y (2011) Paper supercapacitors by a solvent-free drawing method. Energy Environ Sci 4(9):3368–3373
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51203018), the Fundamental Research Funds for the Central Universities (No. 2232015D3-14).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, J., Li, X., Li, X. et al. A flexible carbon electrode based on traditional cotton woven fabrics with excellent capacitance. J Mater Sci 52, 9773–9779 (2017). https://doi.org/10.1007/s10853-017-1161-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1161-z