Abstract
The green-emitting SrSi2O2N2:Eu2+ phosphors with single phase have been successfully synthesized by a simple two-step method. During the first-step process, Sr2SiO4:Eu3+ precursor was formed and emitted red light under UV light excitation. The excessive amount of Si3N4 in reactants promoted the formation of pure SrSi2O2N2 phase when molar ratio of Si3N4/Sr2SiO4 was located in the range of 1.1–1.5. The luminescence intensities of SrSi2O2N2:Eu2+ phosphors were enhanced by Si3N4/Sr2SiO4 molar ratio of 1.1. Phase analysis, luminescence properties and thermal stability of the samples were intensively investigated by X-ray diffraction, SEM, XPS and TGA/DSC. The trivalent europium ions were identified by XPS results on the surface of as-synthesized SrSi2O2N2:Eu2+ particles with approximate theoretical atomic ratio of Eu/Sr, which prevent the further oxidation of Eu2+ to Eu3+ in the bulk structure and evidently improved the thermal stability of the phosphors. For the thermal oxidation mechanism, it was suggested that the oxidation of SrSi2O2N2 to form SrSiO3 layer firstly occurred on the surface after a calcination process in air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, solid-state lighting based on phosphor-converted light-emitting diodes (pc-LEDs) technology has been widely used in general illumination fields due to their high efficiency, long lifetime and environmental friendly characteristics [1,2,3,4,5,6]. However, the early pc-LEDs consist of blue chip, and yellow YAG:Ce3+ phosphor shows a low color rendering index (Ra < 80) and cannot meet the requirement of developing interior lighting [5, 7,8,9]. For pc-LEDs, phosphors play a key role in achieving optimal white light with excellent color rendering index and high correlated color temperature. Therefore, a variety of novel green and red phosphors have been developed to improve the optical properties of pc-LEDs.
Among them, rare-earth-doped nitridosilicates and oxonitridosilicates phosphors are the most likely candidates for general illumination application, which shows high quantum efficiency, small thermal quenching and suitable excitation spectra [5, 10,11,12,13,14,15,16,17,18,19,20,21]. Nowadays, the typical nitridosilicates phosphors—Sr2Si5N8:Eu2+ and (Ca,Sr)AlSiN3:Eu2+ with long wavelength emissions, high quantum efficiency and excellent thermal stability—have been successfully acted as red components in pc-LEDs. As a member of nitridosilicates and oxonitridosilicates phosphors family, SrSi2O2N2:Eu2+ phosphor was firstly developed by Hintzen et al. and could emit yellow–green light under UV and blue light irradiation, which was considered as a promising candidate of green phosphor to improve color rendering index and correlated color temperature for pc-LEDs [22]. Furthermore, Li et al. reported the preparation of SrSi2O2N2:Eu2+ phosphor by using Sr2SiO4 as the precursors. However, an unknown phase also can be observed in XRD patterns for all the samples [23]. Until now, it is still a great challenge to prepare SrSi2O2N2:Eu2+ phosphor with single phase by traditional solid-state reaction or other synthetic methods. The impurity phases in SrSi2O2N2:Eu2+ phosphor will decrease the luminescence intensity and thermal stability. Therefore, it is more significant to synthesize pure phase of SrSi2O2N2:Eu2+ with expected thermal stability.
In this paper, SrSi2O2N2:Eu2+ phosphor with single phase has been successfully prepared by a simple two-step method. The excessive amount of Si3N4 in the second step promoted the formation of pure SrSi2O2N2 phase when molar ratio of Si3N4/Sr2SiO4 was located in the range of 1.1–1.5. The luminescent intensity also reached to maximum with Si3N4/Sr2SiO4 molar ratio of 1.1. It is suggested that the existence of Eu3+ ions on the surface of as-synthesized SrSi2O2N2:Eu2+ phosphor prevents the further oxidation of Eu2+ in bulk structure during the calcination process in air.
Experimental
SrSi2O2N2:xEu2+ (x = 0.01–0.14) phosphors were synthesized using raw materials of SrCO3 (99.9%, Sinopharm, China), SiO2 (Sinopharm, China), α-Si3N4 (Aldrich) and Eu2O3 (99.99%, Aldrich). The samples were prepared by a two-step method according the following equation:
-
1.
\( {\text{SiO}}_{2} + 2{\text{SrCO}}_{3} + x{\text{Eu}}_{2} {\text{O}}_{3} \to {\text{Sr}}_{2} {\text{SiO}}_{4}:2x{\text{Eu}}^{3 + } + 2{\text{CO}}_{2} + 3x/2{\text{ O}}_{2} \)
-
2.
\( {\text{Sr}}_{2} {\text{SiO}}_{4}:2x{\text{Eu}}^{3 + } + {\text{Si}}_{3} {\text{N}}_{4} \to 2{\text{SrSi}}_{2} {\text{O}}_{2} {\text{N}}_{2}:x{\text{Eu}}^{2 + } \)
In the first step, the raw materials of SiO2, SrCO3 and Eu2O3 were weighed stoichiometrically and mixed thoroughly in an agate mortar. Then, the powder mixture was loaded into a furnace and sintered in air for 4 h at 1100 and 1180 °C, respectively. In the second step, the obtained Sr2SiO4:2xEu3+ precursor and excessive amount of Si3N4 were finely ground in agate mortar. The Si3N4/Sr2SiO4 molar ratio of 1.0–1.5 was employed. Subsequently, the powder mixtures were transferred to a tubular furnace and sintered at 1450 °C for 5 h under a flow condition of 3% H2/N2 gas (100 mL/min). After sintering, the samples were cooled with the furnace and the phosphors with green color were finally obtained.
The phase of samples was identified by X-ray powder diffraction (XRD-7000S, Shimadzu) with Cu Kα radiation (λ = 0.15374 nm) operating at 40 kV and 20 mA. The data were collected with a step of 0.02° (2θ) and a count time of 1s per step in continuous scan mode. Photoluminescence (PL) spectra were recorded by a HITACH F-4500 fluorescence spectrophotometer at room temperature. The particle morphology of the phosphors was observed by field emission electron microscopy (FESEM, JSM-7800F, JEOL). The surface elements of powders were analyzed by X-ray photoelectron spectroscopy (Thermofisher ESCALAB 250Xi). Thermogravimetric (TG) and differential scanning calorimetry (DSC) were performed using a thermometric system (Setsys 16/18, Setaram) under air flow in the temperature ranging from 25 to 1000 °C at a heating rate of 10 °C/min.
Results and discussion
Phase identification and luminescence property of precursor
A different temperature (1100 and 1180 °C) was employed in the first step to investigate the phase composition of the precursors. It is shown in Fig. 1 that raw material of SrCO3 (PDF No.: 5-418) can be found in the precursor obtained at 1100 °C and the precursor with pure Sr2SiO4 phase is formed under the temperature of 1180 °C. Eu3+ ions were introduced into the structure of Sr2SiO4 during the first-step process. Therefore, no Eu2O3 phase can be observed in the XRD patterns and the characteristic luminescent spectra (Fig. 2) of Eu3+ can be obtained for the precursor of Sr2SiO4:Eu3+. The excitation spectrum of Sr2SiO4:Eu3+ precursor presents an intense band with maximum at 394 nm by monitoring the 5D0–7F1 transition of Eu3+ ion (587 nm). It is well known that the emission spectra of Eu3+ ion are attributed to 5D0–7F J (J = 0–4) transitions. In Fig. 2b, the emission spectrum of Sr2SiO4:Eu3+ precursor displays the characteristic 5D0–7F1 transition (587 nm) and 5D0–7F2 transition (613 nm) of Eu3+ ion.
Effect of excessive amount of Si3N4 on phase composition and luminescent intensity of Sr0.98Si2O2N2:0.02Eu2+ phosphors
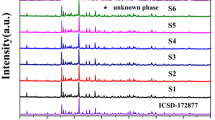
X-ray powder diffraction was performed on the prepared samples to verify the phase purity. Figure 3 shows the standard data of SrSi2O2N2 crystal (ICSD No. 172877) and the representative XRD patterns of SrSi2O2N2:Eu2+ phosphors prepared using the different molar ratio of Si3N4/Sr2SiO4. No impurity phase of Sr2SiO4 or SrSiO3 is observed in Fig. 3a–e. However, there is also a diffraction peak of 2θ = 28.2° marked by filled diamond in Fig. 3a, which has been attributed to unknown phase in other literatures. When the excessive amounts (Si3N4/Sr2SiO4 molar ratio of 1.1–1.5) of Si3N4 were employed in the second step, all the diffraction peaks in Fig. 3b–e can be indexed to the standard data of SrSi2O2N2 which own the triclinic structure with space group P1, a = 7.0802(2) nm, b = 7.2306(2) nm, c = 7.2554(2) nm, α = 88.767(3), β = 84.733(2), γ = 75.905(2) and V = 358.73(2) nm3, Z = 4. Therefore, it is indicated that the obtained phosphors in this case are pure triclinic SrSi2O2N2 phase.
The excitation and emission spectra of Sr0.98Si2O2N2:0.02Eu2+ phosphors prepared with different molar ratio of Si3N4/Sr2SiO4 are presented in Fig. 4. The excitation spectra monitored at 526 nm show a broad band from 300 to 470 nm. The intense green emission bands ~526 nm are observed in the emission spectra under the excitation of 370 nm, which is attributed to 4f 65d–4f 7 transition of Eu2+ ions. With the Si3N4/Sr2SiO4 molar ratio increasing, the luminescence intensity increases firstly and then decreases. The absorption achieves the strongest when Si3N4/Sr2SiO4 molar ratio is 1.1.
Photoluminescence properties and morphological observation of Sr1−x Si2O2N2:xEu2+ phosphors
The concentration of activator plays a key role in the performance of a phosphor. Therefore, it is important to investigate the relationship between Eu2+ concentrations (x) of Sr1−x Si2O2N2:xEu2+ phosphors and photoluminescent emission intensity. A series of Sr1−x Si2O2N2:xEu2+ phosphors with various Eu2+ concentrations (x) were prepared using Si3N4/Sr2SiO4 molar ratio of 1.1. The influence of Eu2+ concentration (x) on luminescence properties of the prepared phosphors is shown in Fig. 5. In Fig. 5a, the emission intensity of the phosphors increases firstly with increasing Eu2+ concentration (x) and reaches the strongest intensity when x = 0.02; then, the emission intensity decreases due to the concentration quenching with a further increasing of x. Figure 5b exhibits the dependence of relative emission intensity and wavelength on Eu2+ concentration (x). The emission bands of the green phosphors ranged from 526 to 537 nm with the changes of x (0.01–0.14).
The particle size and morphology of precursor and phosphor were characterized by a field emission scanning electron microscope (FESEM). Figure 6a indicates that Sr2SiO4:Eu3+ precursors are pellet with particle size of ~1 μm. Polyhedral morphology with particle size of 1–10 μm can be found for SrSi2O2N2:Eu2+ phosphors in Fig. 6b. SrSi2O2N2:Eu2+ phosphors show clear crystal facets and growth habits of layer structure for triclinic SrSi2O2N2 crystal.
Thermal stability analysis
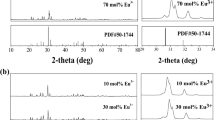
The thermal quenching performance of SrSi2O2N2:Eu2+ phosphor is usually studied as an intrinsic property of phosphor. In contrast to the recoverability of thermal quenching, thermal stability is regarded as plastic performance and cannot be recovered when thermal attack is removed [21, 24]. High thermal stability of a phosphor becomes more significant for its practical application in white LEDs. Thermal degradation usually relates to the valence band of activator and the chemical composition of the host lattice. In this work, the thermal stability of SrSi2O2N2:Eu2+ has been investigated by a calcined method in air for 4 h at temperature of 400 °C, which will be in favor of the understanding of thermal degradation or thermal oxidation of phosphor. As shown in Fig. 7a, the luminescent intensity of SrSi2O2N2:Eu2+ phosphor declines by ~30% after calcination at 400 °C. It has been found that the phase composition (Fig. 7b) and morphologies (Fig. 7c, d) of the samples after calcination at 400 °C are consistent with the ones of as-synthesized samples. Figure 8a shows TG–DSC curves of the as-synthesized SrSi2O2N2:Eu2+ phosphor. It can be seen from TG curves that an appreciable weight gain begins at 800 °C and a massive weight gain appears after 900 °C owing to the oxidation of phosphor. The thermal oxidation temperature is confirmed as 915 °C by extension lines in Fig. 8a. The exothermic variation tendencies in the DSC curves also demonstrate the oxidation process of the phosphor in accordance with the results of TG. Based on the results of TG–DSC, Sr0.95Si2O2N2:0.05Eu2+ phosphor was sintered at 1200 °C in air (denoted as SSON-1200) to investigate the oxidation of phosphor. In Fig. 8b, all diffraction peaks of SSON-1200 sample are attributed to crystalline SrSiO3 phase (PDF card No. 34-0099). Apparently, SrSi2O2N2 was oxidized as SrSiO3 phase during the thermal oxidation process.
XPS was also employed to determine the surface states of as-synthesized Sr0.95Si2O2N2:0.05Eu2+ phosphor (denoted as SSON) and the samples calcined at 400 °C (denoted as SSON-400), as shown in Fig. 9. From Fig. 9a, peaks assigned to core levels of Eu 3d, O 1s, N 1s, Sr 3p, Sr 3d, Si 2p are identified. Two intense peaks at 1164.4 and 1134.8 eV in Fig. 9b are attributed to the core levels of Eu 3d 3/2 and Eu 3d 5/2, respectively, which indicates the oxidation states of europium ions are mainly trivalent both for SSON and SSON-400 samples. Considering that XPS is a surface detection technology and the characteristic 4f 65d–4f 7 transition of Eu2+ ions for the samples, Eu3+ ions are only located on the surface of SSON and SSON-400 particles. As shown in Fig. 9c, the O 1s region for both samples all can be fitted into two peaks centered at 532.1 and 530.3 eV, respectively, which means at least two kinds of oxygen species are present. The peak at 532.1 eV is due to Si–O bands, while the peak at 530.3 eV is associated with Sr–O bands. Then, the relative peak intensity of Sr–O band (PSr–O) and Si–O band (PSi–O) can be calculated by the corresponding fit peak area. The relative peak intensity (0.426) of SSON-400 is a little more than that (0.415) of SSON, meaning larger amount of Sr–O bands in SSON-400. In Fig. 9d, double peaks at 134.8 and 133.1 eV are ascribed to the core levels of Sr 3d 3/2 and Sr 3d 5/2, respectively. The theoretical atomic ratio of Eu to Sr was quantitatively determined as 0.053 for Sr0.95Si2O2N2:0.05Eu2+ phosphor. The Eu/Sr ratios determined by XPS analysis for SSON and SSON-400 samples are 0.052 and 0.051, respectively, closing to the theoretical atomic ratio of 0.053. The results suggest that Eu concentration on the surface of phosphors is consistent with the ones in the bulk.
Thermal oxidation mechanism
As evidenced by the core-level Eu 3d XPS spectra, the trivalent europium ions are identified on the surface of SrSi2O2N2:Eu phosphors. Therefore, europium ions of the phosphors are consisted of Eu3+ ions on the surface and Eu2+ ions in the bulk because SrSi2O2N2:Eu phosphors show the characteristic 4f 65d–4f 7 transition of Eu2+ ions. On the basis of the above-mentioned discussions, the thermal oxidation mechanism of SrSi2O2N2:Eu phosphor is schematically illustrated in Fig. 10. SrSi2O2N2 crystal shows a similar layer structure with BaMgAl10O17:Eu2+ phosphor in which Sr2+ (or Eu2+) are sandwiched in between the SiON3 layers. It has been reported that thermal degradation of BaMgAl10O17:Eu2+ phosphor is due to the oxidation of the divalent europium ions according to the following oxidation process: (i) adsorption of oxygen atoms on the surface of phosphors; (ii) diffusion of the divalent europium ions into the conduction layer; (iii) transfer of an electron from a dopant ion to a adsorbed oxygen ion [25]. Considering the similar lattice structure, SSON phosphor will go through a same thermal oxidation process with BaMgAl10O17:Eu2+ phosphor. Eu2+ ions in the host crystal of SrSi2O2N2 were well protected from oxidation by the existence of Eu3+ ions on the surface of phosphor during a heat treatment process because Eu3+ ions layer has an adverse effect on (i) adsorption of oxygen atoms on the surface of phosphors and (ii) diffusion of the divalent europium ions. Thus, the thermal oxidation of SrSi2O2N2:Eu phosphors in this work is different from the ones reported in other literatures, in which the oxidation of activators appears firstly. In our cases, O2 mainly attacks on the host crystal of SrSi2O2N2 to form the corresponding oxide compound layer (SrSiO3) demonstrated by larger amount of Sr–O bands in SSON-400.
Figure 11 shows the Commission International de L’Eclairage (CIE) chromaticity coordinates and the emitting images for as-synthesized SrSi2O2N2:Eu2+ phosphors with different europium concentration. The intense green emission can be observed for Sr0.98Si2O2N2:0.02Eu2+ phosphor with color coordinates of (x = 0.245, y = 0.659). With increasing Eu2+ contents, the chromaticity coordinates of SrSi2O2N2:Eu2+ phosphors change from green (x = 0.241, y = 0.658) to yellow green region(x = 0.299, y = 0.648) in accordance with the inset images of SrSi2O2N2:Eu2+ phosphors under 370-nm light excitation.
Conclusions
In summary, SrSi2O2N2:Eu2+ phosphors with single phase were conveniently prepared by a two-step method using molar Si3N4/Sr2SiO4 ratio of 1.1–1.5. The luminescent intensity of SrSi2O2N2:Eu2+ phosphors reached to maximum when the molar ratio of Si3N4/Sr2SiO4 was 1.1. The emission bands of the green phosphors ranged from 526 to 537 nm with the increases in Eu concentration. Trivalent europium ions were identified on the surface of the as-synthesized samples with approximate theoretical atomic ratio of Eu/Sr and play a key role to avoid oxidation of Eu2+ ions in the bulk during a heat treatment process. Furthermore, the thermal oxidation of SrSi2O2N2:Eu2+ phosphors was attributed to the formation of the SrSiO3 layer on the surface of SrSi2O2N2 particles.
References
Daicho H, Iwasaki T, Enomoto K et al (2012) A novel phosphor for glareless white light-emitting diodes. Nat Commun 3:1132–1139
Hoppe HA (2009) Recent developments in the field of inorganic phosphors. Angew Chem Int Ed 48:3572–3582
Lin CC, Liu RS (2011) Advances in phosphors for light-emitting diodes. J Phys Chem Lett 2:1268–1277
Piquette A, Bergbauer W, Galler B, Mishra KC (2015) On choosing phosphors for near-UV and blue LEDs for white light. ECS J Solid State Sci 5:R3146–R3159
Pust P, Weiler V, Hecht C et al (2014) Narrow-band red-emitting Sr[LiAl3N4]:Eu2+ as a next-generation LED-phosphor material. Nat Mater 13:891–896
Strobel P, Schmiechen S, Siegert M, Tücks A, Schmidt PJ, Schnick W (2015) Narrow-band green emitting nitridolithoalumosilicate Ba[Li2(Al2Si2)N6]:Eu2+ with framework topologywhjfor LED/LCD-backlighting applications. Chem Mater 27:6109–6115
Li G, Chen J, Mao Z, Song W, Sun T, Wang D (2015) Carbothermal synthesis of CaAlSiN3:Eu2+ red-emitting phosphors and the photoluminescent properties. J Mater Sci Mater Electron 26:10201–10206
Li G, Chen J, Mao Z, Song W, Sun T, Wang D (2016) Atmospheric pressure preparation of red-emitting CaAlSiN3:Eu2+ phosphors with variable fluxes and their photoluminescence properties. Ceram Int 42:1756–1761
Zhang ZW, Liu L, Song ST, Zhang JP, Wang DJ (2015) A novel red-emitting phosphor Ca9Bi(PO4)7:Eu3+ for near ultraviolet white light-emitting diodes. Curr Appl Phys 15:248–252
Smet PF, Botterman J, Van den Eeckhout K, Korthout K, Poelman D (2014) Persistent luminescence in nitride and oxynitride phosphors: a review. Opt Mater 36:1913–1919
Chen WT, Sheu HS, Liu RS, Attfield JP (2012) Cation-size-mismatch tuning of photoluminescence in oxynitride phosphors. J Am Chem Soc 134:8022–8025
Li YQ, de With G, Hintzen HT (2006) Luminescence properties of Ce3+-activated alkaline earth silicon nitride M2Si5N8 (M = Ca, Sr, Ba) materials. J Lumin 116:107–116
Mueller-Mach R, Mueller G, Krames MR et al (2005) Highly efficient all-nitride phosphor-converted white light emitting diode. Phys Status Solidi A 202:1727–1732
Tsai YT, Chiang CY, Zhou W, Lee JF, Sheu HS, Liu RS (2015) Structural ordering and charge variation induced by cation substitution in (Sr, Ca)AlSiN3: Eu phosphor. J Am Chem Soc 137:8936–8939
Yeh CW, Chen WT, Liu RS et al (2012) Origin of thermal degradation of Sr2−x Si5N8: Eu x phosphors in air for light-emitting diodes. J Am Chem Soc 134:14108–14117
Kim HS, Ki Machida, Itoh M, Hanzawa H (2014) Synthesis and luminescence properties of (Sr, Ca)AlSiN3:Eu2+ phosphors under atmospheric-pressure. ECS J Solid State Sci 3:R234–R237
Kirakosyan A, Mnoyan A, Cheong SH, Lee GY, Young Jeon D (2012) Double-color emitting SrSi2O2N2:Eu2+, Yb2+ oxynitridosilicate phosphor for warm white-light emitting diode with highly stable color chromaticity. ECS J Solid State Sci 2:R5–R8
Lee SH, Kim KB, Kim JM, Jeong YK, Kang JG (2013) Highly enhanced luminescence of SrSi2O2N2:Eu2+ phosphors by Mg2+ substitution and their application in pc-LED. Phys Status Solidi A 210:1093–1097
Piao X, Horikawa T, Hanzawa H, Machida K (2006) Characterization and luminescence properties of Sr2Si5N8:Eu2+ phosphor for white light-emitting-diode illumination. Appl Phys Lett 88:161908(01)–161908(03)
Qin J, Lei B, Li J et al (2013) Temperature-dependent long-lasting phosphorescence in SrSi2O2N2:Eu2+. ECS J Solid State Sci 2:R60–R64
Wang CY, Xie RJ, Li F, Xu X (2014) Thermal degradation of the green-emitting SrSi2O2N2:Eu2+ phosphor for solid state lighting. J Mater Chem C 2:2735–2742
Li YQ, Delsing ACA, de With G, Hintzen HT (2005) Luminescence properties of Eu2+-activated alkaline-earth silicon-oxynitride MSi2O2-δN2+2/3δ (M = Ca, Sr, Ba): a promising class of novel LED conversion phosphors. Chem Mater 17:3242–3248
Li X, Zhang R, Cui R, Deng C (2015) Luminescent properties of SrSi2O2N2:Eu2+ by the two-step synthesis. J Alloys Compd 650:470–474
Zhang C, Uchikoshi T, Xie R-J et al (2015) Reduced thermal degradation of the red-emitting Sr2Si5N8:Eu2+ phosphor via thermal treatment in nitrogen. J Mater Chem C 3:7642–7651
Bizarri G, Moine B (2005) On BaMgAl10O17:Eu2+ phosphor degradation mechanism: thermal treatment effects. J Lumin 113:199–213
Acknowledgements
This work was supported by the Excellent Talents Plan Project for Universities of Liaoning Province (LJQ2014055), the Open Project of Key Laboratory for Micro/Nano Technology and System of Liaoning Province (20140401), the General Project of Liaoning Provincial Education Department and the Start-up Funding for Doctoral researchers of Dalian Polytechnic University (61020726).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Cheng, Z., Zhang, Y. et al. Improved luminescence properties and thermal stability of SrSi2O2N2:Eu2+ phosphor with single phase via the formation of Eu3+ on surface structure. J Mater Sci 52, 7605–7614 (2017). https://doi.org/10.1007/s10853-017-0992-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0992-y