Abstract
Graphene aerogel (GA) has wide applications in energy storage, catalysis, and adsorption. However, as a candidate for supercapacitor electrode, it suffers from low specific capacitance. In this paper, we conceived and conducted a two-step strategy to improve the electrochemical properties of GA: First, GA was selective functionalized with p-phenylenediamine. Then PANI was grafted onto GA by in situ polymerization. The results suggest that surface functionalization enhances the interface bonding between GA and PANI and charge transfer during electrode redox. As a result, the obtained GAp/PANI composites, applied as the freestanding electrodes for supercapacitor, exhibit high specific capacitance (810 F/g at 1 A/g, 2.4 times of GA), good rate capability, and extraordinary cycling stability (83.2% of retention at 10 A/g after 10,000 cycles, 1.25 times of PANI/GA), indicating its great potential in the construction of high-performance energy-storage systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the fast development of sustainable and renewable energy storage technology, supercapacitors have been applied as a novel type of energy storage devices. It is well known for high power density, long cycle life, and high efficiency [1, 2]. For the application of supercapacitors, two categories of electrode materials have been widely exploited and investigated in the last decades, that is, electrical double-layer (EDL) capacitor materials and pseudocapacitive materials [3]. EDL capacitor materials normally employ carbon materials, such as active carbon, carbon nanotubes, and graphene. In particular, three-dimensional (3D) graphene, shaped like hydrogel/aerogel [4], foam/sponges [5] and nano-architectures [6], has recently received increasing attention because of its unique structure and properties, such as self-supported intact skeleton, high porosity, high specific surface area, good electrical conductivity, and perfect flexibility [7,8,9]. In this respect, 3D graphene is synthesized by electrochemical reduction in graphene oxide (GO) on a gold foil [10] in the literature and results in high areal capacitance (487 mF cm−2) and excellent cycling stability (retention of 93% after 10,000 cycles). Moreover, 3D graphene has exhibited potential in other prospective applications in catalysis [5, 10], adsorption [11], and biological engineering [12].
However, the application of 3D graphene as the candidate of supercapacitor electrodes is limited due to its lower capacitance. The combination of 3D graphene and pseudocapacitive materials is an effective way to tackle this problem. The first choice of pseudocapacitive materials is probably transition metal oxides, such as MnO2 [13], Ni(OH)2 [14], Co3O4 [15], Fe3O4 [16]. Though these oxides can provide high pseudocapacitance, they suffer from low flexibility and poor interface interactions with 3D graphene. Recently, other pseudocapacitive materials, especially conducting polymers, such as polyaniline (PANI) [17], polypyrrole [18] and polythiophene, come to the attention of researchers. Meng et al. [19] prepared porous 3D graphene using sacrificial template method and then deposed PANI by a chemical oxidation. The obtained composites have high specific capacitance (385 F/g at 0.5 A/g) and good cycling stability. Liu et al. [20] prepared 3D graphene/PANI based on cellulose fibers using a “dipping and drying” method, which demonstrates higher energy storage (464 F/g). In another research, Xu et al. [21] reported a hybrid electrode of hierarchical PANI-modified carbon nanofiber/graphene oxide with specific capacitance of 450.2 F/g at 10 mV/s. However, few studies on the interface modification between 3D graphene and the conducting polymers were reported. Recent researches have shown it plays an important role in improving the electrochemical performances of conducting polymer/graphene composites [19,20,21,22,23,24,25,26].
In present study, we demonstrate a two-step strategy to improve the electrochemical properties of GA, a typical kind of 3D graphene, as a freestanding supercapacitor electrode: selective functionalization of GA with p-phenylenediamine, grafting of PANI onto GA by in situ oxidation polymerization of aniline. The resultant materials were characterized using FESEM, FTIR, TG, XRD, RS, and XPS techniques. Electrochemical properties of these materials in acidic electrolyte were evaluated through CV, GCD, EIS, and cycling stability tests. Compared with GA and GA/PANI, the functionalized GA/PANI shows superior supercapacitor performance and exhibits good prospects in future energy storage and conversion devices.
Materials and methods
Materials
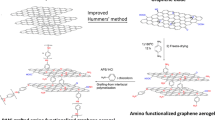
P-phenylenediamine (p-PDA), aniline (An, AR), sodium nitrite (NaNO2, AR), hydrochloric acid (HCl, AR), and ammonium ammonium sulfate (APS, AR) were purchased from Aladdin Industrial Corporation. Ultrapure water was used throughout the experiments. Graphene oxide was synthesized from powdered flake graphite (300 meshes, Qingdao Meilikun, China) by our improved Hummers method [27].
Synthesis of PANI/GA
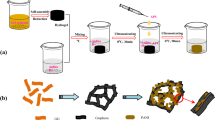
As shown in Fig. 1, the preparation process of GAp/PANI includes three steps, which can be described as follows:
Synthesis of GA
First, 410-mg sodium ascorbate dispersed in 15 mL of H2O was mixed with 20 mL of 3.5 mg/mL GO and sonicated for 30 min. Subsequently, the suspension was transferred into a Teflon-lined stainless steel autoclave and maintained at 140 °C for 6 h. After being cooled down, the resulting graphene hydrogels were washed repeatedly with distilled water, freeze-dried and finally cylinder-like GA foam was obtained. GA was cut into several discs with a thickness of about 1 mm and weight of about 2 mg.
Functionalization of GA
First, 40 mM p-PDA was dissolved in 40 mL of 1 M HCl aqueous solution under stirring for 10 min, and then 40 mM sodium nitrite was added. After that, GA discs were immersed in the solution under low-velocity stirring, allowing the diazonium reaction in ice bath for 6 h. The products were washed repeatedly with distilled water and freeze-dried. The obtained aniline functionalized GA was named as GAp.
Synthesis of GAp/PANI
First, 20 mL of 1 M HCl aqueous solution containing 4 mM aniline was stirred in an ice bath for 10 min, and then GA or GAp discs were immersed. After 20 mL of 0.2 M APS solution was drop-added into the foregoing solution under stirring for 1 h, the polymerization reaction was kept for x hours (x = 3, 6, 9 or 12). The resulting green discs were subsequently raised and freeze-dried, giving the composite samples GAp/PANI-xh. For comparison, the same procedures were conducted for GA without functionalization, and the resulting products were named as GA/PANI-xh. The mass fraction of PANI in GA/PANI composites was obtained by comparing the mass difference between dried GA and GA/PANI composites.
Characterization
The crystal phases of samples were determined by a powder X-ray diffraction system (XRD, PANalytical, X’ Pert PRO, Cu-Ka radiation, λ = 0.15406 nm). The chemical components and structures were analysed using Fourier transform infrared spectroscopy (FTIR; Bruker, IFS66V), Raman spectroscopy (Raman; Renishaw), and X-ray photoelectron spectroscopy (XPS; Kratos, Axis Supra), and XPS spectra were corrected using the C1s line at 284.6 eV. The morphology was observed by a field-emission scanning electron microscopy (FESEM; JEOL, JSM-6701F), and transmission electron microscope (TEM; JEOL, JEM-2000FX). Thermal gravimetric analyzer (TGA; TA, SDT Q600) was used to test the weight loss of samples with a calefactive rate of 5 °C/min under Ar air flow of 60 mL/min.
Electrochemical tests
Cyclic voltammetry (CV), galvanostatic charge/discharge (GCD), and electrochemical impedance spectroscopy (EIS) tests were conducted to evaluate the electrochemical properties of electrode materials based on a three-electrode cell system: GAp/PANI(or GA, GA/PANI) as the freestanding working electrode, a Pt plate as counter electrode, and an SCE as reference electrode, and all the electrochemical tests were conducted in 1M H2SO4. The CV and EIS data were acquired by an electrochemical workstation (PARSTAT2273, AMETEK), while the GCD data were taken by a Battery Test System (Land 2001, Wuhan Kingnuo, China). Mass specific capacitance C (F/g), energy density W (Wh/kg), and power density P (kW/kg) by the following equations [28]:
where I is the constant discharge current (A), m is the mass of electrode materials (g), △t is the discharging time(s), V is the potential (V) with initial and final values of V i and V f, respectively.
Results and discussion
Characterization
Figure 2 shows the FESEM images of (a) GA, (b) GAp, (c) GA/PANI-9h and (d) GAp/PANI-9h, and TEM images of (e) GA/PANI-9h and (f) GAp/PANI-9h. GA derived from hydrothermal reduction in GO has cylinder-like shape (inset of Fig. 2a) and sponge-like microstructure. Within GA, a mass of macropores interconnect, which allows easy access of liquid media [23], which is key to aniline polymerization and electrolyte diffusion. Compared with its origin GA, GAp (Fig. 2b) change little in porous morphology, and the difference lies in the N element mapping (bottom inset of Fig. 2b), which arises from the grafted aniline on the surface of GA. After loaded with PANI, the pores become narrow in both GA/PANI-9h (Fig. 2c) and GAp/PANI-9h (Fig. 2d). Nanometer polyaniline was grown on the surface of graphene uniformly (Fig. 2e, f). But the PANI load of both composites is not high, with a load fraction 10.7 wt% for GA/PANI-9h, 20.9 wt% for GAp/PANI-9h, respectively. This can be explained by the fact that high-concentration GO colloid (3.5 mg/ml) derived GA has dense narrow pores (Fig. 2b), which prevents the effective diffusion of aniline solution within GA. Meantime, the polymerization reaction would be limited due to the dilute monomer (0.1 mM/ml). However, the case can be improved by functionalization of graphene, which can enhance the surface wettability and reaction activity of GA and afford it higher load of PANI.
FESEM images of a GA, b GAp, c GA/PANI-9h, d GAp/PANI-9h, and TEM images of e GA/PANI, and f GAp/PANI. Inset of (a) shows the digital image of GA, insets of (b) show the digital image of GAp (top) and the elemental mapping of GAp (bottom), and insets of (c), (d) and (f) shows the corresponding high-magnification image, respectively
Figure 3a illustrates the FTIR spectra of GO, GA, GAp, PANI, and GAp/PANI-9h. The spectrum of GO showed strong absorption at 3400, 1729, 1400, and 1060 cm−1 due to –OH, –C=O vibration in COOH and C–O vibration in C–OH/C–O–C [27]. A sharp band around 1625 cm−1 might be from the intramolecular H2O. Due to the reduction, most peaks in GA become weak or disappear except for –COOH group, which remains in the edge of graphene planes. In the spectrum of GAp, new bands at 840 and 1147 cm−1 are assigned to the vibration of N–H and C–N, suggesting that –NH2 was grafted on the surfaces of GAp. The resultant GAp/PANI-9h exhibits similar characteristic peaks as compared to the pure PANI. For GAp/PANI-9h, the peaks at 1299, 1246, 1145, and 818 cm−1 are attributed to the C–N stretching vibration of the benzene ring, stretching vibration of the C–N in the polaron structure of PANI, stretching of C=N and out-of-plane bending vibration of C–H in benzene ring, respectively [29]. Compared with GAp, the main bands centered at 3440, 1566, and 1485 cm−1 can be ascribed to N–H stretching, C–C stretching vibration of the quinoid ring and the C–C benzenoid ring in PANI, indicating the presence of PANI [20]. Figure 3b shows the TGA of GO, GA, GAp, and GAp/PANI-9h. The initial weight loss occurs at about 150 °C, which is correlated with the pyrolysis of the bound water. This is followed by a significant weight loss between 200 and 300 °C, indicating the pyrolysis of the oxidized groups of GO and GA, grafted molecule of GAp and PANI bonded on GA [27]. The order of thermal stability is GAp/PANI-9h < GO < GA < GAp.
Figure S1 gives the XRD patterns of GO, GAp, GAp/PANI-9h. GO has a characteristic diffraction peak at 2θ = 10.31°, corresponding to the (110) interplanar spacing of 7.3 Å [27, 30], and GAp is characterized by the peak at 23.2° (d(002) = 3.7 Å), which indicates the formation of a poorly ordered graphite-like material (d(002) = 3.4 Å for graphite). For GAp/PANI-9h, the crystalline peaks at about 20° and 25° can be assigned to (020) and (002) reflections of PANI in its polyaniline salt form, while two broad diffraction peaks around 25° present the (002) facets of the hexagonal graphitic results from GAp [30]. Figure S2 shows the Raman spectra of GO and GAp, where D band at 1360 cm−1 and G band at 1574 cm−1 can be attributed to the conversion of a sp2-hybridized carbon to a sp3-hybridized carbon, and the in-plane bond-stretching motion of the pairs of C sp2 atoms, respectively [27]. For GO and GAp, the intensity ratio D band to G band I D/I G = 0.94, 1.12, respectively. The increased I D/I G of GA relative to GO indicates the realization of deoxygenation in chemically reduced graphene.
XPS was employed to determine the element and bond composition of GO, GA, GAp, and GAp/PANI-9h. The survey spectra (Fig. 4a) confirm that the main elements C (C1s) and O (O1s) resulting from all samples, and N element (N1s) only from GAp and GAp/PANI-9h samples [31]. As is expected, GO has higher O1s peak than GA, and GAp/PANI has higher N1s peak than GAp. In C1s spectrum of GO (top of Fig. 4b), four types of covalently bonded carbon can be resolved as sp2 carbon (C–C/C=C, 284.6 eV), epoxy/hydroxyl groups (C–O, 286.6 eV), carbonyl groups (C=O, 287.8 eV), and carboxyl groups (O–C=O, 289.5 eV), respectively [27, 32]. After reduction in GO, the C–O bonds are greatly reduced, so C1s spectra of GA, GAp, and GAp/PANI-9h(middle and bottom of Fig. 4b) shift to lower binding energy. In C1s spectra of GAp and GAp/PANI-9h, new C–N bond can be isolated at 286.8 eV among the deconvoluted peaks, indicating the successful bonding of N element in GAp and GAp/PANI-9h. In contrast, C–N bond could not be isolated from the C1s spectrum of GA. The N1s spectra (Fig. 4c) of GAP and GAP/PANI-9h can be resolved into four peaks located at 398.8, 399.6, 400.3, and 401.7 eV, corresponding to benzenoid amine (=N–), quinoid amine (–NH–), nitrogen cationic radical (NH+), and amino positive ion (NH2 +=) [33], respectively. Due to the presence of PANI, GAP/PANI has much higher NH2 + = peak than GAP.
To interpret the formation process of GAp/PANI, we propose a mechanism description, as shown in Fig. 5. First, hydrothermal reduction in GO constructs the 3D framework of GA, drove by the interactions such as hydrophobic–hydrophilic and π–π stacking interactions [10], and some carboxyl remain in the edge of basal plane of graphene; second, the p-PDA functionalization of GA introduces covalent aniline in basal plane of graphene through diazonium reaction and leads to GAp; third, beginning with those active cites, major functionalized aniline and few carboxyl, PANI molecule produced by oxidization polymerization of aniline monomers heterogeneously nucleate and grow, and finally form the PANI/GAp composites. Due to the improvement in surface wettability and reaction activity, GAp can absorb and load higher amount of PANI relative to GA by in situ polymerization, affording it large pseudocapacitance as a supercapacitor electrode.
Electrochemical properties
The CV and GCD curves of GA, GAp, GA/PANI-8h, and GAp/PANI-8h were determined to evaluate the electrochemical properties. Figure 6a shows GA and GAp share similar and smooth CV loops, which indicates the EDL capacitance is most contributive, while the functionalization of GA has not significant effect on the capacitance. When GA and GAp are coupled with PANI, the CV curves observe a new couple of redox peaks from the pseudocapacitive PANI [34], and GAp/PANI-8h has a much larger loop area or specific capacitance than GA/PANI-8h, which can be attributed to the enhanced interface bonding and PANI load within GAp. Figure 6b illustrates the CV curves of GAp/PANI obtained for different reaction time (x = 3, 6, 9, 12 h). It shows that with the increase in reaction time, the loop area first increases and finally decreases, and reaches the maximum value at x = 9 h, which corresponds to the largest specific capacitance. For the GAp/PANI-9h, we further investigated the charge/discharge performance (Fig. 6c). The GCD curves appear nonlinear and symmetrical. As EDL capacitance often behaves linearly during charge/discharge, the nonlinearity indicates that there exists pseudocapacitance in electrode materials. Furthermore, the symmetry of the GCD curve implies that electrode materials work rather stably, owing to the supporting of 3D GA. As the current density increases, the charge/discharge time decreases. With a current density of 5 A/g, the charge/discharge finished fast within 80 s. With a current density of 0.5 A/g, a specific capacitance of 905 F/g is obtained. This value is superior over the reported results [35,36,37]. To further evaluate and compare the rate performance, we plot the specific capacitance versus current density in Fig. 6d for GA, GA/PANI-9h and GAp/PANI-9h. Clearly, the specific capacitance stored by GAp/PANI-9h dominates over the other two. For example, this value at 1 A/g is 810 F/g for GAp/PANI-9h, and 335 F/g for GA and 466 F/g for GA/PANI-9h. Second, the capacitance declines slowly cross a wide range of current density, suggesting that the materials are of good rate capability. For GAp/PANI-9h, the capacitance only loses 25% of its initial value when the current density increases from 0.5 to 5 A/g. So it is necessary for the functionalization of GA and coupling of PANI in GA as dual strategies to improve the capacitance performance of GA.
EIS is an effective tool to judge the charge transport phenomena of electrode materials [38]. Nyquist (Z′–Z″) plot of GA, GAP, GA/PANI-9h, and GAP/PANI-9h was measured from 10 kHz to 0.1 Hz. Theoretically, the Z′–Z″ curves have two segments, a low frequency region (right) and a high frequency region (left), and the latter often emerges as a semicircle. Moreover, the semicircle is characterized by a starting point standing for the equivalent series resistances R i and a diameter for the electrode/electrolyte interface charge transfer resistance R CT [37, 39]. Figure 7a shows as supported by the conducting GA, R i of GA, GAP, and GAP/PANI-9h is low, but that of GA/PANI is maximum (0.55 Ω). Furthermore, the corresponding R CT is also small, and GA/PANI still has the maximum value, indicating weak interface bonding resists the charge transfer. Figure 7b plots the imaginary capacitance as a function of frequency. From the location of peaks, one can determine the characteristic frequency f 0 and time constant τ = 1/2π f 0. It shows τ = 1.79, 12.2, 16 and 2.65 s for GA, GAP, GA/PANI-9h, and GAP/PANI-9h, respectively, so GAP/PANI-9h has lower value of τ than GA/PANI-9h, further suggesting surface functionalization can improve the kinetics of charge transfer cross the electrodes.
Figure 8a evaluates the cycling performance of capacitance for GA, GA/PANI-9h and GAP/PANI-9h, by GCD at a current density of 10 A/g for 10,000 cycles [27, 40]. The retention of capacitance for GA, GA/PANI-9h, and GAP/PANI-9h is 98.18, 67, and 83.2%, respectively, that is, the cycling stability of GA/PANI-9h is far less than that of the other two. This might be because of the partial detachment of PANI from GA substrate during the long-term cycling [41], due to the absence of strong interface bonding between PANI and GA. In contrast, thanks to the strong bonding between functionalized GA and PANI, GAp/PANI exhibits high cycling stability. Figure 8b presents Ragone plot of the three samples. It shows after loaded with PANI, the energy density of GA noticeably increases, and GAp/PANI-9h possesses both high energy density and high power density. Further, we assembled a symmetric supercapacitor based on GAp/PANI-9h (inset of Fig. 8b), which can lighten a LED lamp for 5 min. The tested results (Fig. S3, Supplementary) show it has a specific capacitance of 211.2 F/g, about 25% of that value of three-electrode system, and a long circle life, consistent with the three-electrode system.
Conclusions
In summary, in present research we demonstrated a new routine to prepare high-performance GA/PANI composites as the freestanding electrodes for supercapacitor. The routine includes two key steps: (1) selective functionalization of GA with p-phenylenediamine via the diazonium reaction, (2) the grafting of the pseudocapacitive PANI onto GA by in situ oxidation polymerization. The obtained materials were characterized using various instrumental methods, such as FESEM, TEM, FTIR, TG, RS, and XPS, and their formation mechanisms and electrochemical properties were investigated in details. The results suggest that owing to the application of surface functionalization, the interface bonding between GA and PANI, the load of PANI and the electrode/electrolyte interface charge transfer during electrode redox were enhanced. Therefore, as compared to GA and nonfunctionalized GA/PANI, the functionalized GA/PANI composites exhibit superior supercapacitor performance. Our work not only highlights the importance of interface modification in composite design, but also provides a proper routine for the development of high-performance supercapacitors.
References
Zhao YH, Zhang YF, Wu ZK, Bai SL (2016) Synergic enhancement of thermal properties of polymer composites by graphene foam and carbon black. Compos B Eng 84:52–58
Long JW, Bélanger D, Brousse T, Sugimoto W, Sassin MB, Crosnier O (2011) Asymmetric electrochemical capacitors-stretching the limits of aqueous electrolytes. MRS Bull 36(07):513–522
Zhang Z, Xiao F, Guo Y, Wang S, Liu Y (2013) One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities. ACS Appl Mater Interfaces 5(6):2227–2233
Chi K, Zhang Z, Xi J, Huang Y, Xiao F, Wang S, Liu Y (2014) Freestanding graphene paper supported three-dimensional porous graphene–polyaniline nanocomposite synthesized by inkjet printing and in flexible all-solid-state supercapacitor. ACS Appl Mater Interfaces 6(18):16312–16319
Chen Z, Ren W, Gao L, Liu B, Pei S, Cheng HM (2011) Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat Mater 10(6):424–428
Chung DDL (2016) A review of exfoliated graphite. J Mater Sci 51(1):554–568. doi:10.1007/s10853-015-9284-6
Choi BG, Yang M, Hong WH, Choi JW, Huh YS (2012) 3D macroporous graphene frameworks for supercapacitors with high energy and power densities. ACS Nano 6(5):4020–4028
Wang ZL, Xu D, Wang HG, Wu Z, Zhang XB (2013) In situ fabrication of porous graphene electrodes for high-performance energy storage. ACS Nano 7(3):2422–2430
Zhang R, Cao Y, Li P, Zang X, Sun P, Wang K, Zhong M, Wei J, Wu D, Kang F, Zhu H (2014) Three-dimensional porous graphene sponges assembled with the combination of surfactant and freeze-drying. Nano Res 7(10):1477–1487
He S, Chen W (2015) 3D graphene nanomaterials for binder-free supercapacitors: scientific design for enhanced performance. Nanoscale 7(16):6957–6990
Li W, Gao S, Wu L, Qiu S, Guo Y, Geng X, Chen M, Liao S, Zhu C, Gong Y, Xu J, Wei X, Sun M, Liu L, Long M (2013) High-density three-dimension graphene macroscopic objects for high-capacity removal of heavy metal ions. Sci Rep 3:2125
Wang W, Guo S, Ruiz I, Ozkan M, Ozkan CS (2013) Three dimensional graphene-CNTs foam architectures for electrochemical capacitors. ECS Trans 50(43):37–44
Zhang Z, Xiao F, Qian L, Xiao J, Wang S, Liu Y (2014) Facile synthesis of 3D MnO2–graphene and carbon nanotube-graphene composite networks for high-performance, flexible, all-solid-state asymmetric supercapacitors. Adv Energy Mater 4(10):867–872
Fujisawa K, Cruz-Silva R, Yang KS, Kim YA, Hayashi T, Endo M, Terrones M, Dresselhaus MS (2014) Importance of open, heteroatom-decorated edges in chemically doped-graphene for supercapacitor applications. J Mater Chem A 2(25):9532–9540
Rakhi RB, Chen W, Cha D, Alshareef HN (2012) Substrate dependent self-organization of mesoporous cobalt oxide nanowires with remarkable pseudocapacitance. Nano Lett 12(5):2559–2567
Wang H, Xu Z, Yi H, Wei H, Guo Z, Wang X (2014) One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high performance anode materials for supercapacitors. Nano Energy 7:86–96
Zhang Z, Chi K, Xiao F, Wang S (2015) Advanced solid-state asymmetric supercapacitors based on 3d graphene/MnO2 and graphene/polypyrrole hybrid architectures. J Mater Chem A 3(24):12828–12835
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 2:797–828
Meng Y, Wang K, Zhang Y, Wei Z (2013) Hierarchical porous graphene/polyaniline composite film with superior rate performance for flexible supercapacitors. Adv Mater 25(48):6985–6990
Liu L, Niu Z, Zhang L, Zhou W, Chen X, Xie S (2014) Nanostructured graphene composite papers for highly flexible and foldable supercapacitors. Adv Mater 26(28):4855–4862
Xu D, Xu Q, Wang K, Chen J, Chen Z (2013) Fabrication of free-standing hierarchical carbon nanofiber/graphene oxide/polyaniline films for supercapacitors. ACS Appl Mater Interfaces 6(1):200–209
Wang W, Guo S, Penchev M, Ruiz I, Bozhilov KN, Yan D, Ozkan M, Ozkan CS (2013) Three dimensional few layer graphene and carbon nanotube foam architectures for high fidelity supercapacitors. Nano Energy 2(2):294–303
Fan H, Zhao N, Wang H, Xu J, Pan F (2014) 3D conductive network-based free-standing PANI-RGO-MWNTs hybrid film for high-performance flexible supercapacitor. J Mater Chem A 2(31):12340–12347
Chen W, He Y, Li X, Zhou J, Zhang Z, Zhao C, Gong C, Li S, Pan X, Xie E (2013) Facilitated charge transport in ternary interconnected electrodes for flexible supercapacitors with excellent power characteristics. Nanoscale 5(23):11733–11741
Liang M, Zhi L (2009) Graphene-based electrode materials for rechargeable lithium batteries. J Mater Chem 19(33):5871–5878
Meng C, Liu C, Fan S (2009) Flexible carbon nanotube/polyaniline paper-like films and their enhanced electrochemical properties. Electrochem Commun 11(1):186–189
Yu H, Zhang B, Bulin C, Li R, Xing R (2016) High-efficient synthesis of graphene oxide based on improved hummers method. Sci Rep 6:36143
Mai LQ, Minhas-Khan A, Tian X, Hercule KM, Zhao YL, Lin X, Xu X (2013) Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat Commun 4:2923
Salvatierra RV, Cava CE, Roman LS, Zarbin AJ (2013) ITO-free and flexible organic photovoltaic device based on high transparent and conductive polyaniline/carbon nanotube thin films. Adv Funct Mater 23(12):1490–1499
Domingues SH, Salvatierra RV, Oliveira MM, Zarbin AJ (2011) Transparent and conductive thin films of graphene/polyaniline nanocomposites prepared through interfacial polymerization. Chem Commun 47(9):2592–2594
Salvatierra RV, Oliveira MM, Zarbin AJ (2010) One-pot synthesis and processing of transparent, conducting, and freestanding carbon nanotubes/polyaniline composite films. Chem Mater 22(18):5222–5234
McAllister MJ, Li JL, Adamson DH, Schniepp HC, Abdala AA, Liu J, Herrera-Alonso MiliusLD, Car R, Prud’homme RK, Aksay IA (2007) Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem Mater 19(18):4396–4404
Wang S, Ma L, Gan M, Fu S, Dai W, Zhou T, Sun X, Wang H, Wang H (2015) Free-standing 3D graphene/polyaniline composite film electrodes for high-performance supercapacitors. J Power Sources 299:347–355
Lee T, Yun T, Park B, Sharma B, Song HK, Kim BS (2012) Hybrid multilayer thin film supercapacitor of graphene nanosheets with polyaniline: importance of establishing intimate electronic contact through nanoscale blending. J Mater Chem 22(39):21092–21099
Hyder MN, Lee SW, Cebeci FÇ, Schmidt DJ, Shao-Horn Y, Hammond PT (2011) Layer-by-layer assembled polyaniline nanofiber/multiwall carbon nanotube thin film electrodes for high-power and high-energy storage applications. ACS Nano 5(11):8552–8561
Yu P, Zhao X, Huang Z, Li Y, Zhang Q (2014) Free-standing three-dimensional graphene and polyaniline nanowire arrays hybrid foams for high-performance flexible and lightweight supercapacitors. J Mater Chem A 2(35):14413–14420
Khoh WH, Hong JD (2013) Layer-by-layer self-assembly of ultrathin multilayer films composed of magnetite/reduced graphene oxide bilayers for supercapacitor application. Colloids Surf A 436:104–112
Augustyn V, Simon P, Dunn B (2014) Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ Sci 7(5):1597–1614
Kulkarni SB, Patil UM, Shackery I, Sohn JS, Lee S, Park B, Jun S (2014) High-performance supercapacitor electrode based on a polyaniline nanofibers/3D graphene framework as an efficient charge transporter. J Mater Chem A 2(14):4989–4998
Sarker AK, Hong JD (2012) Layer-by-layer self-assembled multilayer films composed of graphene/polyaniline bilayers: high-energy electrode materials for supercapacitors. Langmuir 28(34):12637–12646
Zhi M, Xiang C, Li J, Li M, Wu N (2013) Nanostructured carbon–metal oxide composite electrodes for supercapacitors: a review. Nanoscale 5(1):72–88
Acknowledgements
This work is supported by the National Research Foundation of China (No. 51164026), the Inner Mongolia Natural Science Fund of china (2012MS0710), and Talent Incubation Funding of School of Materials and Metallurgy (No. 2014CY012).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bulin, C., Yu, H., Ge, X. et al. Preparation and supercapacitor performance of functionalized graphene aerogel loaded with polyaniline as a freestanding electrode. J Mater Sci 52, 5871–5881 (2017). https://doi.org/10.1007/s10853-017-0823-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0823-1