Abstract
The Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures with different Cd concentrations were prepared using spin coating technique on GaN substrate. The structural properties of Cu2Zn1−x Cd x SnS4/GaN were investigated by X-ray diffraction (XRD) and field emission-scanning electron microscope. XRD studies indicated that kesterite phase of Cu2ZnSnS4 and stannite phase of Cu2CdSnS4 were formed. The optical properties were studied through photoluminescence technique, and indicated that the band gap shifted as Cd concentration increases from 1.75 eV in Cu2ZnSnS4 to 1.65 eV in Cu2CdSnS4. The electrical characterization of the Ag/n-GaN/Cu2Zn1−x Cd x SnS4/Ag diode through current to voltage (I–V) characterization showed the highest photoresponse of (value if any) at Cu2Zn0.4Cd0.6SnS4 composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stoichiometric Cu2–II–IV–VI4 (II = Zn, Cd, Hg; IV = Si, Ge, Sn; VI = S, Se, Te) chalcogenide semiconductors have drawn the increasing interest during the past few decades due to their potential applications in photovoltaic [1–4] and thermoelectric [5, 6] devices. Especially, ongoing efforts have been devoted to the Cu2ZnSnS4 (CZTS) solar cells in view of their power conversion efficiencies, even as high as 12.0 % [3]. The fabrication techniques for CZTS thin films become flourishing correspondingly, e.g. sputtering [7], thermal evaporation [8, 9], chemical vapour deposition [10], atomic layer deposition [11], electrodeposition [4], spray coating [2] and spray pyrolysis [12, 13].
Theoretical calculations indicated that the best fit with solar spectrum and the optimal band gap for absorber materials is around 1.4 eV. To reach the ideal band gap for CZTSSe-based single-junction solar cells, the ratio of S/Se is modified in several studies [14–16]. The synthesis of Cu2Cd x Zn1−x SnS4 (0 ≤ x ≤ 1) (CZCTS) alloy by sol–gel method has been accomplished [17] to achieve the goal of bandgap engineering in Cu2ZnSnS4-based solar cells. Ibraheam et al. [17] have reported that the optical band gap of CZCTS alloy can be also modified continuously from 1.55 to 1.09 eV as Cd varies from 0 to 1.
The low-cost Cu2CdSnS4 nanocrytal thin film with a stannite structure has been successfully fabricated by Zhao et al. [18]. The selenized Cu2CdSn(S,Se)4 thin film shows large densely packed grains and has a suitable bandgap value of 1.01 eV. The Cu2CdSn(S,Se)4 thin-film solar cell with a proof-of-concept power conversion efficiency of 3.1 % was fabricated. Recently, Guo et al. [19] have fabricated Cu2ZnSnS4 (CZTS) thin films by sulphurization of direct current and pulse reverse co-electrodeposited CZTS precursors on Mo-coated glass substrates. The CZTS thin film synthesized using pulse reverse co-electrodeposition exhibits homogeneous and large grains, Cu-poor and Zn-rich compositions and free of Cu2S secondary phase compared with that obtained from direct current co-electrodeposition. By using pulse reverse co-electrodeposition, the performance of pen junction is improved with low diode quality factor (A) and reverse saturation current density (J 0), and the CZTS device first reaches an efficiency of 6.28 % (V oc = 609 mV, J sc = 18.4 mA/cm2, FF = 56.1 %) threshold, which is higher than that of 4.69 % (V oc = 561 mV, J sc = 16.4 mA/cm2, FF = 50.9 %) using direct current co-electrodeposition. This result offers a novel research direction for preparing high-efficiency CZTS thin-film solar cells.
Till now, there are no reports on the optical and morphological properties of Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures deposited on n-type GaN substrates; therefore, in this study, high-quality Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures were deposited on GaN substrates by spin coating technique to study the structural properties given by X-ray diffraction (XRD) and field emission-scanning electron microscope (FE-SEM), and optical properties of Cu2Zn1−x d x SnS4 quinternary alloy nanostructures by photoluminescence (PL), in addition to investigating the electrical properties of Ag/n-GaN/Cu2Zn1−x Cd x SnS4/Ag diode at x = 0, 0.6, 1 for photodetector applications through (I–V) characterization.
Experimental process
Spin coating technique is used to deposit the Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures onto GaN (0001) substrate. First, a solution of Cu2Zn1−x Cd x SnS4 precursors was prepared from copper(II) chloride dihydrate, zinc(II) chloride dihydrate, tin(II) chloride dihydrate thiourea, 2-methoxyethanol (2-metho) and monoethanolamine (MEA). The 2-metho and MEA were used as solvent and stabilizer, respectively. The molar ratio of MEA to solution of Cu2Zn1−x Cd x SnS4 was at 0.5. The precursor solution (0.3 M) was stirred at 50 °C for 3 h to completely dissolve the metals during stirring, and after adding thiourea, the milk solution became yellow and transparent. The molar ratio of Cu, (Zn + Cd), Sn and S in the solution was 2:1:1:4. To obtain solutions with different Cd concentrations (x), the molar ratio of Cd to (Zn + Cd) in the solution varied to give x values of 0, 0.6, 0.8 and 1. The precursor solutions were mixed based on the required ratio of the following formula Cu 2+2 (Zn1−x Cd x )2+ Sn4+ S4 8−. This means that the solution consisted of two parts of Cu and one part of (Zn,Cd) depending on the value of x, one part Sn and four parts S. The solution was then dropped onto a GaN (0001) substrate (20 mm × 20 mm × 1 mm) rotating at 2500 rpm for 30 s. After deposition by the spin coating, the nanostructures were dried at 250 °C for 80 min on a hot plate. The coating and drying processes were repeated seven times to obtain a film of 1 µm thickness.
The crystal structure of the Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures was examined by X-ray diffraction (XRD; PW 1710 X-ray diffractometer, Phillips, USA) with Cu Kα radiation (λ = 1.54 Å). The optical properties were measured at room temperature using UV–Visible spectrophotometry (Lambda 950, Perkin Elmer, USA) and photoluminescence spectroscopy (Jobin Yvon model HR 800 UV system, Jobin Yvon, USA) at room temperature using a He–Cd laser (λ = 325 nm). Surface morphology and grain size were investigated by field emission-scanning electron microscopy (FE-SEM) (NOVA NANO SEM 450, USA). The topography of the films was characterized by atomic force microscopy (AFM) (SII SPI 3800N Probe, Seiko Instruments Inc., USA). Thickness measurement was carried out using the weight method given by
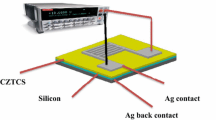
where t is thickness, ∆m is a difference of substrate weight (substrate after deposition—substrate before deposition), A is area of sample and ρ is the density of deposited material. Finally, heat treatment was conducted in an elevator furnace under N2 gas flow (5 % gas atmosphere) for 1 h at 300 °C, and after annealing, the samples were cooled to below 40 °C in the chamber. After that, Ag metal contacts were formed on Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures with Cd concentration equals 0, 0.6, 1. PVD-HANDY/2STE (Vaksis Company, USA) vacuum thermal evaporation in the pressure of 4.5 × 105 Torr was used for deposition on GaN, and the contacts were formed in the form of zig-zag with 5 mm length and 100 nm thickness as shown in Fig. 1. The contact area of the diode was found to be 3.14 × 102 cm2. For the current to voltage (I–V) characterization, the fabricated device was connected in parallel with the Kiethly (2400 source meter, USA). The reading was recorded from −6 to 6 V. For current to time (I–t) analysis, the device was connected in series with the multimeter and the value was recorded by switching the LED on and off. The wavelength and power of the LED used for conducting the experiment were 490 nm wavelength and 3 mW power, respectively.
Results and discussion
The typical XRD pattern of the Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures is shown in Fig. 2. It can be seen that the major diffraction peaks at 2θ = 17.46°, 22.93°, 28.26°, 34.39°, 39.85° and 44.13° can be attributed to the (101), (110), (112), (004), (102) and (213) plans of kesterite phase of Cu2ZnSnS4 and stannite phase of Cu2CdSnS4. Owing to the presence of four or five elements in the materials, there was a possibility for the secondary phases like SnS, attributed to the (110) was observed at 2θ = 41.99° corresponding to JSPDS cards (00-001-1244). According to the Debye–Scherrer formula, (112) peak was calculated to increase the accuracy, and the average crystallite size of the CZCTS nanostructures can be presented in Table 1. The relatively intense and sharper (112) and (200) diffraction peaks were observed for nanostructures deposited at x = 0.6 and 1. However, when the concentration of Cd was further increased to 0.6 and 1, the intensity of diffraction peaks increases, which indicates that the crystallinity increase with increasing in concentration of Cd. The peak (112) was shifted to the lower angle side with increasing Cd concentration in the CZCTS solid solutions, which was attributed to the increasing lattice constant. This was due to the radius of Cd ion (1.53 Å) which is larger than that of Zn (1.33 Å) as supported by previous work [17]. The simplest possibility is that Cd substitutes other metals at their sites in crystal lattice of Cu2Cd x Zn1−x SnS4 quinternary alloy nanostructures. As the theoretical calculated substitution energies of Cd atoms at Cu, Sn and Zn atom sites in CZCTS lattice are E sub (CdCu) = 0.69 eV, E sub (CdSn) = 1.07 eV and E sub (CdZn) = 0.53 eV [20], the most likely is the isoelectronic substitution of Cd at the Zn site. Lattice constants a and c were calculated from XRD data for the (112) plane, which are given in Table 1.
Figure 3 shows FE-SEM micrographs of Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures with Cd concentration equals (a) 0, (b) 0.6 and (c) 1. From FE-SEM images, the obtained Cu2Zn1−x Cd x SnS4 layer on GaN substrate was found to be homogeneous with good adherence to the substrate. Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures are clearly discernible, indicating an isolated grain growth at the surface. With the addition of Cd, the shape of the grains changed significantly (Fig. 3). The grain shape becomes rounder as well as bigger for 0.6. The presence of nanowire rod shaped when x = 1 might lead to the formation of more densely packed nanostructures with less porosity.
The elemental analysis of Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures with different Cd concentrations was investigated using EDAX and their compositions are illustrated in Fig. 4. The presence of Cu, Zn, Cd, Sn and S elements is confirmed by EDAX analysis. Furthermore, it is also interesting to note that the composition of Zn in Cu2Zn1−x Cd x SnS4 varies upon increasing the Cd concentration. The nanostructures were copper poor and zinc rich at x (0, 0.6) concentrations [23, 24].
Figure 5 represents the three-dimensional topographic AFM images Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures with Cd concentration equals 0, 0.6 and 1. For a detailed study on average roughness properties of Cu2Zn1−x Cd x SnS4 with Cd concentration, line profiles were recorded. From the line profile analysis, the calculated average roughness values are 3.56, 4.356 and 5.167 nm for x = 0, 0.6, 1, respectively. Minimum average surface roughness value is 3.56 nm. It was found that the incorporation of Cd results in the increasing of surface roughness.
PL spectra of CZCTS quinternary alloy nanostructures with different Cd concentrations measured at room temperature are shown in Fig. 6. PL spectra consist of one broad asymmetric PL band at 1.75 eV in Cu2ZnSnS4 and at 1.65 eV in Cu2CdSnS4 as given in Table 1. A shift of PL band of Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures towards highest wavelengths with increasing Cd concentration is observed in the region 0 ≤ x ≤ 1. This shifting is due to the substitution of Zn atoms with Cd atoms to produce a lower energy gap [25, 26].
Figure 7 shows that the I–V characteristics from −6 to 6 V of Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures with Cd concentration values x = 0, 0.6, 1 measured in the dark and under illuminating were 490 nm and 3 mW. The difference between I dark and I ph is increased in Cu2Zn0.4Cd0.6SnS4 heterojunction compared with Cu2ZnSnS4 (x = 0) heterojunction. The photosensitivities of the heterojunction were calculated by
Based on the I–V curve (Fig. 7) and Eq. (2), the I dark and I ph and S ph are listed in Table 2. Figure 7 shows that the photosensitivity increases with increasing Cd concentration to 99.9982 for x = 0.6 and decreased to 99.4826 for x = 1. There is no significant difference in photosensitivity when the concentration of Cd increases.
To confirm the better performance of the heterojunction and reproducibility of Ag/GaN/Cu2Zn1−x Cd x SnS4/Ag device, it was examined by cyclically switching the white light on and off. Figure 7 shows the I ph as a function of time intervals (I–t) of Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures at x = 0, 0.6, 1. I ph sharply increases/decreases to reach the maximum/minimum under 490 nm light on/off. The Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures at x = 0.6 (Fig. 7b) show better performance than at x = 0, 1 (Fig. 8). The result indicated an improvement with increasing Cd concentration, x = 0.6. The I ph/I dark ratio was 2.6, 3.6 and 3 for x = 0, 0.6 and 1, respectively, as given in Table 3. The calculated response time (t Res) and decay time (t Rec) using 490 nm and 3 V show that all values decreased with increasing of Cd concentration indicating an improved photoresponse. The performance of Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures shows t Res (0.02654) and t Rec (0.01064) for x = 0.6 (Table 3).
Conclusion
The Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures were prepared on n-GaN substrate using spin coating technique. XRD results revealed that the as-prepared CZCTS exhibits impurity-free kesterite phase of Cu2ZnSnS4 and stannite phase of Cu2CdSnS4 in polycrystalline nature with the crystallite sizes between 44.69 and 58.22 nm. The band gap was found to decrease with increasing Cd concentrations. It was found that the incorporation of Cd results in an increasing of surface roughness of Cu2Zn1−x Cd x SnS4 nanostructures from 3.56 to 4.356 nm. Under illumination densities, the n-GaN/CZCTS junction exhibited good photoconductivity. The I on/I off ratios confirmed the photosensitivity of the produced structure. Photocurrent measurements showed the highest photoresponse for Cu2Zn0.4Cd0.6SnS4 quinternary alloy nanostructures.
References
Cao YY, Denny MS, Caspar JV, Farneth WE, Guo QJ, Ionkin AS, Johnson LK, Lu MJ, Malajovich I, Radu D, Rosenfeld HD, Choudhury KR, Wu W (2012) High-efficiency solution-processed Cu2ZnSn(S, Se)4 thin-film solar cells prepared from binary and ternary nanoparticles. J Am Chem Soc 134:15644–15647
Carrete A, Shavel A, Fontane X, Montserrat J, Fan JD, Ibanez M, Saucedo E, Perez-Rodriguez A, Cabot A (2013) Antimony-based ligand exchange to promote crystallization in spray-deposited Cu2ZnSnSe4 solar cells. J Am Chem Soc 135:15982–15985
Winkler MT, Wang W, Gunawan O, Hovel HJ, Todorov TK, Mitzi DB (2014) Optical designs that improve the efficiency of Cu2ZnSn(S, Se)4 solar cells. Energy Environ Sci 7:1029–1036
Ahmed S, Reuter KB, Gunawan O, Guo L, Romankiw LT, Deligianni H (2012) A high efficiency electrodeposited Cu2ZnSnS4 solar cell. Adv Energy Mater 2:253–259
Fan FJ, Yu B, Wang YX, Zhu YL, Liu XJ, Yu SH, Ren ZF (2011) Colloidal synthesis of Cu2CdSnSe4 nanocrystals and hot-pressing to enhance the thermoelectric figure-of-merit. J Am Chem Soc 133:15910–15913
Ibanez M, Zamani R, LaLonde A, Cadavid D, Li WH, Shavel A, Arbiol J, Morante JR, Gorsse S, Snyder GJ, Cabot A (2012) Cu2ZnGeSe4 nanocrystals: synthesis and thermoelectric properties. J Am Chem Soc 134:4060–4063
Scragg JJ, Ericson T, Fontane X, Izquierdo-Roca V, Perez-Rodriguez A, Kubart T, Edoff M, Platzer-Bjorkman C (2014) Rapid annealing of reactively sputtered precursors for Cu2ZnSnS4 solar cells. Prog Photovolt 22:10–17
Schubert BA, Marsen B, Cinque S, Unold T, Klenk R, Schorr S, Schock HW (2011) Cu2ZnSnS4 thin film solar cells by fast coevaporation. Prog Photovolt 19:93–96
Shin B, Gunawan O, Zhu Y, Bojarczuk NA, Chey SJ, Guha S (2013) Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber. Prog Photovolt 21:72–76
Ramasamy K, Malik MA, O’Brien P (2011) The chemical vapor deposition of Cu2ZnSnS4 thin films. Chem Sci 2:1170–1172
Thimsen E, Riha SC, Baryshev SV, Martinson ABF, Elam JW, Pellin MJ (2012) Atomic layer deposition of the quaternary chalcogenide Cu2ZnSnS4. Chem Mater 24:3188–3196
Nie L, Liu S, Chai Y, Yuanm R (2015) Spray pyrolysis deposition and photoresponse of Cu2CdSnS4 thin films. J Anal Appl Pyrol 112:363–368
Daranfed W, Aida MS, Attaf N, Bougdira J, Rinnert H (2012) Cu2ZnSnS4 thin films deposition by ultrasonic spray pyrolysis. J Alloys Compd 542:22–27
Timmo K, Altosaar M, Raudoja J, Muska K, Pilvet M, Kauk M, Varema T, Danilson M, Volobujeva O, Mellikov E (2010) Sulfur-containing Cu2ZnSnSe4 monograin powders for solar cells. Sol Energy Mater Sol Cells 94:1889
Cao Y, Xiao Y, Jung J-Y, Um H-D, Jee S-W, Choi HM, Bang JH, Lee J-H (2013) Highly electrocatalytic Cu2ZnSn(S1−x Se x )4 counter electrodes for quantum-dot-sensitized solar cells. Appl Mater Interfaces 5:479
Wang Y-P, Levcenco S, Dumcenco DO, Huang Y-S, Ho C-H, Tiong K-K (2013) Composition dependent band gaps of single crystal Cu2ZnSn(S x Se1−x )4 solid solutions. Solid State Phenom 194:139–144
Ibraheam AS, Al-Douri Y, Hashim U, Ghezzar MR, Addou A (2015) Waleed K. Ahmed. Cadmium effect on optical properties of Cu2Zn1−x Cd x SnS4 quinternary alloys nanostructures. Sol Energy 114:39–50
Zhao W, Wang G, Tian Q, Huang L, Gao S, Pan D (2015) Solution-processed Cu2CdSn(S, Se)4 thin film solar cells. Sol Energy Mater Sol Cells 133:15–20
Guo Min, Zhu Xiurong, Li Hejun (2016) Comparative study of Cu2ZnSnS4 thin film solar cells fabricated by direct current and pulse reverse co-electrodeposition. J Alloy Compd 657:336–340
Maeda T, Nakamura S, Wada T (2012) First-principles study on Cd doping in Cu2ZnSnS4 and Cu2ZnSnSe4. Jpn J Appl Phys 51:10NC11–10NC16
Suehiro S, Horita K, Kumamoto K, Yuasa M, Tanaka T, Fujita K, Shimanoe K, Kida T (2014) Solution-processed Cu2ZnSnS4 nanocrystal solar cells: efficient stripping of surface insulating layers using alkylating agents. J Phys Chem C 118:804–810
Nie L, Liu S, Chai Y, Yuan R (2015) Spray pyrolysis deposition and photoresponse of Cu2CdSnS4 thin films. J Anal Appl Pyrolysis 112:363–368
Kahraman S, Cetinkaya S, Cetinkara HA, Guder HS (2014) Effects of diethanolamine on sol–gel–processed Cu2ZnSnS4 photovoltaic absorber thin films. Mater Res Bull 50:165–171
Fischereder A, Rath T, Haas W, Amenitsch H, Albering J, Meischler D, Larissegger S, Edler M, Saf R, Hofer F, Trimmel G (2010) Investigation of Cu2ZnSnS4 formation from metal salts and thioacetamide. Chem Mater 22:3399–3406
Lee KH, Kim HY, Bang HJ, Jung YH, Lee SG (2003) The change of bead morphology formed on electrospun polystyrene fibers. Polymer 44:4029–4034
Luque PA, Quevedo-Lopez MA, Olivas A (2013) Influence of deposition time on ZnS thin film growth over SiO2 and glass substrates. Mater Lett 106:49–51
Acknowledgements
Y.A. would like to thank University Malaysia Perlis for grant No. 9007-00185. K.D.V. would like to acknowledge U.G.C., New Delhi, India for providing financial assistance in the form of Major Research Project [Code: 42-856/2013(SR)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibraheam, A.S., Al-Douri, Y., Hashim, U. et al. Fabrication, analysis and characterization of Cu2Zn1−x Cd x SnS4 quinternary alloy nanostructures deposited on GaN. J Mater Sci 51, 6876–6885 (2016). https://doi.org/10.1007/s10853-016-9975-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-9975-7