Abstract
It has been found that the partial substitution of Fe, Al, Ag, Cu, Ni, V, or Cr for Be effectively enhances the glass-forming ability of Ti–Zr–Be ternary alloy. The influence of different alloying elements on crystallization kinetics of Ti41Zr25Be34 alloy has been investigated by isothermal and isochronal differential calorimetry combined with X-ray diffraction. Among the selected alloying elements, Fe, Al, Cu, Ni, or Cr can enhance the thermal stability, while the Ag- and V-containing alloys exhibit a slightly narrower supercool liquid region. The crystallization of all the eight alloys proceeds through at least two exothermic events. The apparent activation energies of glass transition and crystallization have been determined based on the Kissinger equation. The substitution of Al, Ag, Cu, or Ni for Be does not change the product of the primary crystallization of the base alloy. However, with the addition of beta stabilized elements (Fe, V, and Cr), the formation of β-Ti as primary phase is promoted. The overall nucleation and growth characteristics of the parent, Al-, Ag-, Cu-, Ni-, V-, and Cr-additive alloys, are similar. Compared with the other seven alloys, Ti41Zr25Be28Fe6 alloy exhibits much larger Avrami exponents with an average value of 5.26, indicating a high-dimensional growth with increasing nucleation rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lightweight bulk metallic glasses (BMGs), which are represented by Al- [1, 2], Mg- [3, 4], and Ti-based BMGs [5–8], have attracted much attention due to the modern concepts for lightweight construction. However, the glass-forming ability (GFA) of Al-based BMGs is very poor while most of Mg-based BMGs are very brittle. In this sense, Ti-based BMGs seem more promising materials to be used in industry fields such as aerospace. In the past decades, a series of Ti-based BMGs, such as Ti–Cu–Ni [9], Ti–Zr–Cu–Ni–Sn [10], Ti–Zr–Cu–Ni–Be [11], Ti–Zr–Cu–Pd–Sn [12], and Ti–Zr–Hf–Cu–Ni–Si–Sn [13], have been successfully developed. However, in order to improve the GFA, most of the available Ti-based BMGs always contain a large portion of heavy elements (e.g., Cu, Pd, etc.), resulting in the decrease of specific strength.
Compared with other developed Ti-based BMGs, Ti–Zr–Be ternary alloys [14, 15] possess relatively high specific strength because of the addition of light element Be. However, the critical size for glass formation of Ti–Zr–Be alloys is no more than 6 mm [15], which is relatively small compared with that of some well-known ternary BMGs (e.g., Zr–Ni–Al [16], Mg–Cu–Y [17], etc.). In order to solve this problem, alloying method, which is widely used to improve the properties of alloys, has been adopted to enhance the GFA of Ti41Zr25Be34 alloy. In previous work, it has been found that the GFA of Ti41Zr25Be34 BMG can be greatly improved by replacing Be by Al [18] or some transitional elements (e.g., Fe [19], Ag [20], Cu [21], Ni [22], V [14], and Cr [15]). Moreover, the optimized alloying content for most of the selected elements is around 6 at.%. Unfortunately, the underlying mechanisms of GFA improvement of Ti41Zr25Be34 alloy induced by alloying have not been fully understood yet.
As the glass formation is a competing process between liquid phase and competing crystalline phases, research on the crystallization kinetics of is helpful to further understand the GFA [23–25]. In this study, the crystallization behaviors of Ti41Zr25Be34 and seven representative quaternary BMGs with different fourth elements have been investigated by X-ray diffraction (XRD) and differential scanning calorimetry (DSC) under both isochronal and isothermal conditions. Based on the experimental results, the roles of alloying elements on crystallization kinetics of Ti41Zr25Be34 alloy have been exposed.
Experimental procedure
Master ingots with nominal compositions of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Fe, Al, Ag, Cu, Ni, V, and Cr) were prepared by arc melting high purity (>99.9 wt%) constituent elements under a Ti-gettered argon atmosphere. From the ingots, cylindrical alloy rods with different diameters were prepared by copper mold casting method. The amorphous nature of as-cast samples was ascertained by X-ray diffraction (XRD, Rigaku D/max-RB) with Cu Kα radiation. Thermal analysis was carried out in a differential scanning calorimeter (DSC, NETZSCH 404 F1 Pegasus). The DSC system was calibrated for temperature and enthalpy using indium and zinc standards. Continuous heating was carried out at different heating and cooling rates (5, 10, 20, and 40 K min−1) with a flow of purified argon gas using a platinum pan with alumina liner. In the case of isothermal annealing, the samples were put in aluminum pan and heated to the selected temperature at a heating rate of 150 K min−1, and then held until the crystallization completed. In order to eliminate the sample size effect, the DSC samples were cut from ϕ3 mm as-cast rods and the weight of each sample was between 10 and 20 mg. In order to find out the crystalline phases corresponding to different crystallization steps, 3 mm diameter samples were heated to the selected temperatures in DSC at a heating rate of 20 K min−1. After cooling, the annealed samples were polished to remove the oxide layer on the surface and examined by XRD.
Results and discussion
Figure 1 shows the XRD patterns of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Fe, Al, Ag, Cu, Ni, V, and Cr) rods with their critical diameters (D c). All the patterns consist of only a broad diffraction halo peak without any sharp Bragg peak, indicating the amorphous structure of the as-cast rods. The critical diameter of ternary Ti41Zr25Be34 alloy (base alloy) is only 5 mm. However, with the addition of different fourth elements, the glass-forming ability can be improved in varying degrees. Among all the seven selected elements, Cu and Ni are the best choices for improving the GFA as Ti41Zr25Be28Cu6 and Ti41Zr25Be28Cu6 alloys possess a relatively large critical diameter of 15 mm, which is three times as that of the base alloy. Even for the worst element V, the critical size can also be enhanced from 5 to 6 mm. The above results confirm that alloying method is really effective to enhance the GFA of Ti–Zr–Be ternary alloy.
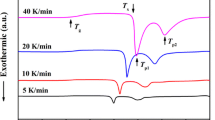
Figures 2 and S1 show the continuous heating DSC curves of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Fe, Al, Ag, Cu, Ni, V, and Cr) glassy alloys measured at the heating rates of 5, 10, 20, and 40 K min−1. The characteristic temperatures such as T g (the glass transition temperature), T x (the onset temperature of crystallization), and T p1 (the first crystallization peak temperature) are marked on the DSC curves and their values are listed in Table 1. It is worthwhile to note that the crystallization behavior can be changed by the addition of some alloying elements. As shown in Fig. 2, the base alloy has a two-stage crystallization with a relatively sharp second exothermic event. With the addition of Al, Ag, Cu, V, or Cr, the corresponding quaternary alloys exhibit similar crystallization behavior. In turn, the Fe bearing alloy has a two-stage crystallization with a relatively sharp first peak and the Ni containing alloy shows three-stage crystallization. It can also be found that the supercooled liquid region (defined as ΔT x = T x –T g) increases with the addition of Fe, Al, Cu, Ni, or Cr. Among all the alloys listed in Table 1, Ti41Zr25Be28Fe6 alloy possesses the widest supercooled liquid region of over 100 K, which is larger than that of most developed Ti-based BMGs. However, the GFA of Ti41Zr25Be28Fe6 alloy is not as good as Ti41Zr25Be28Cu6 and Ti41Zr25Be28Ni6 alloys which possess a smaller ΔT x value. Moreover, ΔT x decreases slightly with Ag or V addition but the GFA has been improved. In this sense, although ΔT x is always used as an indicator of the GFA for BMGs, the relationship between ΔT x and GFA of Ti–Zr–Be–M glassy alloys seems weak. Similar phenomena have been reported in some other alloy systems [26].
From Figs. 2, S1 and Table 1, it can also be seen that the characteristic temperatures of all the listed alloys are shifted to higher temperature side with increasing heating rates. The activation energy of the glass transition or crystallization reaction under continuous heating condition can be calculated by the Kissinger equation [27]:
where β is the heating rate, E is the apparent activation energy for the process, R is the gas constant, T represents the characteristic temperature (such as T g, T x , and T p1), and C is a constant. Figure 3 shows the Kissinger plots of glassy alloys calculated by T g, T x and T p1, respectively. The calculated activation energy of the glass transition and crystallization are listed in Table 1. For all the eight alloys, E g is higher than E x and E p1, indicating that the glass transition needs more activation energy than the crystallization reaction. It has been presumed that E x and E p1 represent the activation energy for nucleation and growth, respectively [28]. As shown in Table 1, E p1 of Ti41Zr25Be28Ag6 alloy is slightly higher than E x , which means the growth process of the crystalline phases is more difficult than the nucleation process. Correspondingly, the other seven alloys are just the reverse. From Table 1, we can also find that E g, E x , and E p1 of the present alloy series have no obvious relationship with ΔT x and GFA. Among all the listed eight alloys, Ti41Zr25Be28V6 alloy has the largest E g, while Ti41Zr25Be28Ag6 alloy has the largest E x and E p1. However, the critical diameter D c and ΔT x of these two alloys are not the largest according to the experimental results provided in Fig. 1 and Table 1. It has been reported that Ag addition enhances the glass-forming ability and enlarge the supercooled liquid region of Cu–Zr [29] and Cu–Zr–Al [30] alloys but decreases the activation energy of crystallization, which is attributed to a microstructural heterogeneity induced by Ag addition. In this sense, the addition of alloying elements may also induce inhomogeneity in Ti–Zr–Be alloy.
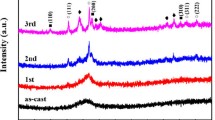
In order to find out the precipitation phases corresponding to different crystallization stages, samples were heated to the extrapolated end temperatures of different crystallization peaks, respectively, at a heating rate of 20 K min−1, and cooled to room temperature. Figures 4 and S2 illustrate the crystallization behaviors of these eight BMGs. The corresponding crystalline phases of different alloys have been summarized in Table 2. For Ti41Zr25Be34, Ti41Zr25Be28Al6, Ti41Zr25Be28Ag6, and Ti41Zr25Be28Cu6 alloys, the primary phase is indexed as α-Ti2Zr. After complete crystallization beyond the highest crystallization peak temperature, more crystallization products such as Be2Zr and α-Ti (α-TiZr for Ti41Zr25Be28Ag6 alloy) can be detected. For Ti41Zr25Be28Ni6 alloys, the first and second crystallization events are associated with the precipitation of α-Ti2Zr and Be2Zr phases, while α-Ti is detected after the third exothermic event. However, for Ti41Zr25Be28Fe6, Ti41Zr25Be28V6, and Ti41Zr25Be28Cr6 alloys, the primary phase is no longer α-Ti2Zr but β-Ti. This is because that Fe, V, and Cr are all typical beta-stabilizing elements which can lower the alpha-to-beta transition temperature. After the second crystallization event, instead of the Be2Zr and α-Ti phases which are the crystalline phases for Ti41Zr25Be28V6 and Ti41Zr25Be28Cr6 samples, BeTi and α-Ti2Zr phases are detected in Ti41Zr25Be28Fe6 sample. According to Zhang’s research [14], for Ti–Zr–Be alloy system, the ternary eutectic consisting of two solution phases and one intermetallics exhibits lower GFA than that of the one solid solution and two intermetallics. So it is reasonable that Ti41Zr25Be28Fe6 alloy exhibits better GFA compared with Ti41Zr25Be28V6 and Ti41Zr25Be28Cr6 alloys. The above results indicate that the crystallization products especially the primary phase of Ti–Zr–Be ternary alloy can be selected according to demand by adding different alloying elements, and this is meaningful for preparing bulk metallic glass composite with good mechanical properties.
In order to further understand the crystallization kinetics of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Fe, Al, Ag, Cu, Ni, V or Cr) glassy alloys, the isothermal kinetics of the samples was characterized by annealing the samples at T x -20 K and T x -30 K (T x here is measured at a heating rate of 20 K min−1). The isothermal DSC curves of Ti41Zr25Be34 alloy are shown in Fig. 5. The isothermal DSC curves of the other seven alloys are shown in Fig. S3 for reasons of simplicity. All DSC curves show a single exothermic peak after certain incubation time. With the increase of annealing time, it can be found that for all the Ti-based alloys, the incubation time decreases and the crystallization process becomes fast, which is in good agreement with previous reports [31–33]. It is also notable that Ti41Zr25Be28Fe6 alloy has a backward-shifted crystallization shape which means the crystallization rate is relatively low in the initial stage, while the other seven alloys have a forward-shifted bell shape. Based on the assumption that the fraction area of the crystallization peak is proportional to the crystallized volume fraction, the relationship between the crystallization volume fraction and annealing time during isothermal process can be obtained and the plots are shown in Fig. 6. All the curves display a typical sigmoidal trend. Except Ti41Zr25Be28Fe6 BMG, the alloys with higher GFA (e.g., Ti41Zr25Be28Ni6, Ti41Zr25Be28Cu6, and Ti41Zr25Be28Ag6 alloys) exhibits relatively long incubation time and broader crystallization peak, indicating their retarded crystallization process.
Details of the crystallization mechanism of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Fe, Al, Ag, Cu, Ni, V, or Cr) during isothermal annealing at T x -30 K can be analyzed the Johnson–Mehl–Avrami (JMA) equation [34]:
where x is the crystallization volume fraction, t is the annealing time, n is the Avrami exponent which reflects the crystallization mechanisms of nucleation and growth, τ is the incubation time for crystallization, and k is a reaction rate constant. Figure 7 shows the JMA plots. As the incubation time of these Ti-based alloy is relatively short, only the data for 0.1 < x < 0.9 are plotted in order to minimize the experimental error inherent in the early and late stages of the experiments. Although the corresponding JMA plots of the rest alloys are nearly straight lines, the JMA plot of Ti41Zr25Be28Fe6 alloy does not have a single slope, indicating the crystallization process of Ti41Zr25Be28Fe6 alloy is more complex than the other seven alloys. The average Avrami exponent n of Ti41Zr25Be34, Ti41Zr25Be28Al6, Ti41Zr25Be28Ag6, Ti41Zr25Be28Cu6, Ti41Zr25Be28Ni6, Ti41Zr25Be28V6, and Ti41Zr25Be28Cr6 alloys are 1.97, 2.07, 2.20, 2.32, 2.32, 1.82, and 2.25, respectively.
As the crystallization mechanism of Ti41Zr25Be28Fe6 alloy changes dramatically during the process, the local Avrami exponent n(x) is introduced and defined as [35]
The variation of the local Avrami exponent of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Fe, Al, Ag, Cu, Ni, V or Cr) alloys at T x -30 K is shown in Fig. 8. In the case of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Al, Ag, Cu, Ni, V or Cr) alloys, the values of Avrami exponent at the early stage of the crystallization (x = 0.1) are 2.45, 2.58, 2.55, 2.68, 2.62, 2.29, and 2.73, respectively. With increasing crystallized volume fraction x, the n values of these seven alloys decrease to about 1.60 in the final stage (x = 0.9). On the other hand, the local Avrami exponent of Ti41Zr25Be28Fe6 alloy shows strong dependence on x with an average value of 5.26, which is much larger than those of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Al, Ag, Cu, Ni, V, or Cr) alloys.
The Avrami exponent n can be interpreted as [36]
where a is a parameter related with the nucleation rate (a = 0 for a zero nucleation rate, 0 < a < 1 for a decreasing nucleation rate with time, a = 1 for a constant nucleation rate and a > 1 for an increasing nucleation rate). b is a parameter showing the dimension of the growth (b = 1, 2, and 3 for one-, two-, and three-dimensional growth, respectively). c is a parameter showing the type of growth (c = 1 for interface-controlled growth and c = 0.5 for diffusion-controlled growth). In this sense, the crystallization behavior of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Al, Ag, Cu, Ni, V, or Cr) alloys is mainly dominated by three-dimensional diffusion-controlled growth with a decreasing nucleation rate. Ti41Zr25Be28Fe6 alloy shows completely different crystallization behavior from the other alloys. The local Avrami exponent increases dramatically and then slightly decrease as the crystallized volume fraction x increases with a large value of 5.26, implying that the crystallization involves a transient nucleation process with an increasing nucleation rate [29]. Surface-induced abnormal grain growth with fractal dimensionality is also a possible reason [37].
Conclusions
The isochronal and isothermal crystallization behaviors of Ti41Zr25Be34 and Ti41Zr25Be28M6 (M = Fe, Al, Ag, Cu, Ni, V, or Cr) glassy alloys have been studied using differential scanning calorimetry. It is indicated that the addition of Fe, Al, Cu, Ni, or Cr obviously enhances the thermal stability while the addition of Ag and V degrades the thermal stability slightly. No obvious relationship between the activation energy of glass transition (or crystallization) and the glass-forming ability has been observed. Unlike Al, Ag, Cu, and Ni, the addition of Fe, V and Cr in Ti41Zr25Be34 BMG leads to the change of primary crystalline phase from α-Ti2Zr phase to β-Ti phase. The isothermal crystallization of Ti41Zr25Be34 alloy is governed by three-dimensional diffusion-controlled growth with a decreasing nucleation rate. With the addition of Al, Ag, Cu, Ni, V, and Cr, there is no fundamental change in the overall nucleation and growth characteristics. However, for Ti41Zr25Be28Fe6 alloy, the local Avrami exponent shows strong dependence on crystallized volume fraction with a large average value of 5.26, implying a more complex crystallization process.
References
Yang H, Wang JQ, Li Y (2007) Glass formation and microstructure evolution in Al-Ni-RE (RE = La, Ce, Pr, Nd and misch metal) ternary systems. Philos Mag 87:4211–4228
Sun BA, Pan MX, Zhao DQ, Wang WH, Xi XK, Sandor MT, Wu Y (2008) Aluminum-rich bulk metallic glasses. Scr Mater 59:1159–11621
Zheng Q, Cheng S, Strader JH, Ma E, Xu J (2007) Critical size and strength of the best bulk metallic glass former in the Mg-Cu-Gd ternary system. Scr Mater 56:161–164
Park ES, Lee JY, Kim DH (2005) Effect of Ag addition on the improvement of glass-forming ability and plasticity of Mg-Cu-Gd bulk metallic glass. J Mater Res 20:2379–2385
Kim YC, Kim WT, Kim DH (2004) A develop of Ti-based bulk metallic glass. Mater Sci Eng A 375–377:127–135
He G, Eckert J, Hagiwara M (2004) Glass-forming ability and crystallization behavior of Ti-CU-Ni-Sn-M (M = Zr, Mo, and Ta) metallic glasses. J Appl Phys 95:1816–1820
Gong P, Wang X, Shao Y, Chen N, Liu X, Yao KF (2013) A Ti-Zr-Be-Fe-Cu bulk metallic glass with superior glass-forming ability and high specific strength. Intermetallics 43:177–181
Jiang JZ, Hofmann D, Jarvis DJ, Fecht HJ (2014) Low-density high strength bulk metallic glasses and their composites: a review. Adv Eng Mater 17:761–780
Wang YL, Xu J (2008) Ti(Zr)-Cu-Ni bulk metallic glasses with optimal glass-forming ability and their compressive properties. Metall Mater Trans A 39:2990–2997
Xie KF, Yao KF, Huang TY (2010) A Ti-based bulk glassy alloy with high strength and good glass-forming ability. Intermetallics 18:1837–1841
Guo FQ, Wang HJ, Poon SJ, Shiflet GJ (2005) Ductile titanium-based glassy alloy ingots. Appl Phys Lett 86:091907
Zhu SL, Wang XM, Inoue A (2008) Glass-forming ability and mechanical properties of Ti-based bulk glassy alloys with large diameters of up to 1cm. Intermetallics 16:1031–1035
Huang YJ, Shen J, Sun JF, Yu XB (2007) A new Ti-Zr-Hf-Cu-Ni-Si-Sn bulk amorphous alloy with high glass-forming ability. J Alloys Compd 427:171–175
Zhang Y, Zhang WG, Lin JP, Hao GJ, Chen GL, Liaw PK (2010) Glass-forming ability and competitive crystalline phases for lightweight Ti-Be-based alloys. Metall Mater Trans A 41:1670–1676
Duan G, Wiest A, Lind ML, Kahl A, Johnson WL (2008) Lightweight Ti-based bulk metallic glasses excluding late transition metals. Scr Mater 58:465–468
Li YH, Zhang W, Dong C, Qiang JB, Makino A, Inoue A (2010) Formation and mechanical properties of Zr-Ni-Al glassy alloys with high glass-forming ability. Intermetallcs 18:1851–1855
Ma H, Zheng Q, Xu J, Li Y, Ma E (2005) Doubling the critical size for bulk metallic glass formation in the Mg-Cu-Y ternary system. J Mater Res 20:2252–2255
Gong P, Yao KF, Shao Y (2012) Lightweight Ti-Zr-Be-Al bulk metallic glasses with improved glass-forming ability and compressive plasticity. J Non-Cryst Solids 358:2620–2625
Gong P, Yao KF, Shao Y (2012) Effects of Fe addition on glass-forming ability and mechanical properties of Ti-Zr-Be bulk metallic glass. J Alloys Compd 536:26–29
Zhao SF, Shao Y, Gong P, Yao KF (2014) A centimeter-sized quaternary Ti-Zr-Be-Ag bulk metallic glass. Adv Mater Sci Eng 2014:192187
Zhao SF, Chen N, Gong P, Yao KF (2015) New centimeter-sized quaternary Ti-Zr-Be-Cu bulk metallic glasses with large glass-forming ability. J Alloy Compd 647:533–538
Zhao SF, Gong P, Li JF, Chen N, Yao KF (2015) Quaternary Ti-Zr-Be-Ni bulk metallic glasses with large glass-forming ability. Mater Des 85:564–573
Patel A, Pratap A (2012) Kinetics of crystallization of Zr52Cu18Ni14Al10Ti6 metallic glass. J Therm Anal Calorim 107:159–165
Wang T, Yang XH, Li Q (2014) Effect of Cu and Nb additions on crystallization kinetics of Fe80P13C7 bulk metallic glasses. Thermochim Acta 579:9–14
Gong P, Yao KF, Ding HY (2015) Crystallization kinetics of TiZrHfCuNiBe high entropy bulk metallic glass. Mater Lett 156:146–149
Lu ZP, Liu CT (2002) A new glass-forming ability criterion for bulk metallic glasses. Acta Mater 50:3501–3512
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Wang HR, Gao YL, Min GH, Hui XD, Ye YF (2003) Primary crystallization in rapidly solidified Zr70Cu20Ni10 alloy from a supercooled liquid region. Phys Lett A 314:81–87
Song KK, Gargarella P, Pauly S, Ma GZ, Kühn U, Eckert J (2012) Correlation between glass-forming ability, thermal stability, and crystallization kinetics of Cu-Zr-Ag metallic glass. J Appl Phys 112:063505
Sung DS, Kwon OJ, Fleury E, Kim KB, Lee JC, Kim DH, Kim YC (2004) Enhancement of the glass forming ability of Cu-Zr-Al alloys by Ag addition. Met Mater Int 10:575–579
Qiao JC, Pelletier JM (2011) Crystallization kinetics in Cu46Zr45Al7Y2 bulk metallic glass by differential scanning calorimetry. J Non-Cryst Solids 357:2590–2594
Jung HY, Stoica M, Yi SH, Kim DH, Eckert J (2014) Crystallization kinetics of Fe76.5-x C6.0Si3.3P8.7Cu x (x = 0, 0.5, and 1 at. pct) bulk amorphous alloy. Metall Mater Trans A 46:2415–2421
Hua NB, Chen WZ, Liu XL, Yue F (2014) Isochronal and isothermal crystallization kinetics of Zr-Al-Fe glassy alloys: effects of high-Zr content. J Non-Cryst Solids 388:10–16
Avrami M (1941) Granulation, phase change, and microstructure kinetics of phase change. III. J Chem Phys 9:177–184
Calka A, Radlinski AP (1988) Decoupled bulk and surface crystallization in Pd85Si15 glassy metallic alloys: description of isothermal crystallization by a local value of the Avrami exponent. J Mater Res 3:59–66
Ranganathan S, Heimendahl MV (1981) The three activation energies with isothermal transformations: applications to metallic glasses. J Mater Res 16:2401–2404
Pratap A, Raval KG, Gupta A, Kulkarni SK (2000) Nucleation and growth of a multicomponent metallic glass. Bull Mater Sci 23:185–188
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51271095).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, P., Wang, X. & Yao, K. Effects of alloying elements on crystallization kinetics of Ti–Zr–Be bulk metallic glass. J Mater Sci 51, 5321–5329 (2016). https://doi.org/10.1007/s10853-016-9835-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-9835-5