Abstract
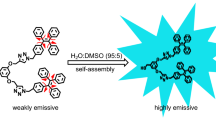
In this work, novel dendritic zinc phthalocyanines showing both molecular NIR fluorescence and aggregation-induced emission characteristics have been successfully synthesized via a two-step reaction on the basis of bisphthalonitrile precursors with different electron donor–conjugation–acceptor (D–π–A) structures. The luminescent properties of the resultant dendritic zinc phthalocyanines were fully investigated in N, N-dimethylformamide (DMF) solution, in DMF/H2O mixed solvent, in the solid state as well as polymethylmethacrylate composite film. The dendritic zinc phthalocyanines substituted with hydroquinone, naphthalenediol, or dihydroxybiphenyl moieties exhibited an unusual luminescence changes from highly near infrared (NIR) emitting in molecular state to strongly blue fluorescence in aggregated state as well as solid state, where the maximum fluorescent emission wavelength and highest quantum yield were detected from dendritic zinc phthalocyanines containing naphthalenediol and dihydroxybiphenyl groups, respectively. On the contrary, dendritic zinc phthalocyanines bearing diphenylmethane or bisphenol A units only exhibit NIR fluorescent emission in their molecular state. The distinct fluorescent emissions for these dendritic zinc phthalocyanines are rationalized in the framework of molecular configuration and electron density distribution as clarified by density functional theory (DFT) calculation. Moreover, the well-organized three-dimensional microspheres composed of abundant rod-like building blocks were discovered via the self-assembly of dendritic zinc phthalocyanine molecules in DMF/H2O (50/50 vol%) mixture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic fluorescent materials have attracted enormous attention in many fields ranging from biological imaging [1, 2], chemical sensors [3], and optoelectronic devices [4] mainly because of their excellent properties including versatile synthetic tailorability and high fluorescence quantum yield. Generally, the conventional organic fluorophores should be used in their dilute solution, since their fluorescent emission would be declined or even fully quenched when they were aggregated or existed in solid state, resulting to the so-called notorious aggregation-caused quenching (ACQ) effect, which is mainly resulted from the formation of non-photoactive species (excimers, H-type aggregates, etc.) [5, 6]. Thus, the ACQ effect of organic fluorophores would strongly hinder their potential applications in fluorescent bioprobes, data storage, chemical sensory systems, and organic light-emitting diodes (OLED) [7,8,9,10,11,12]. Therefore, many research groups have attempted to overcome these inherent disadvantages through chemical and physical approaches to improve the fluorescence quantum yield in aggregated and solid state.
In 2001, the Tang’s team firstly reported the aggregation-induced emission (AIE) for the silole compound 1-methyl-1,2,3,4,5-pentaphenylsilole showing stronger fluorescence in the aggregated state compared to its molecular state in pure solution [13]. The AIE mechanism has been lately rationalized as the restriction of intramolecular rotation that normally dissipates energy of excited fluorophores via various non-radiation channels. Afterward, the tetraphenylethene, 8,8a-dihydrocyclopenta[α]indene, benzene-1,3,5-tricarboxamide, conjugated poly(diphenylacetylene)s, and other hexaphenylsilole derivatives were found with AIE characteristic and have been applied in many cutting edge fields, such as fluorescent pH sensors, biological DNA probes, polarized light emitters, and multi-responsive nanomaterials [14,15,16,17,18]. Thanks to these flourishing applications, increasing efforts have been devoted to design and synthesize enhanced AIE fluorophores including BF2 fluorescent dyes [19], 1,1-Dicyano-2,2-bis(4-dimethylaminophenyl)ethylene [20], 4,4′-(9-oxo-9H-fluorene-2,7-diyl)dibenzaldehyde [21], (1,3-dimethyl)barbituric acid-functionalized anthracenes [22], and α-cyanostilbene functionalized tetraphenyl imidazole derivatives [23]. However, the majority of these AIE fluorophores are non-luminescent in molecular state due to the presence of diverse non-radiative channels. Therefore, the exploration of new-type fluorophores that exhibit both molecular emission and AIE-enabled aggregated or solid-state fluorescence is of great importance for even wider practical applications.
Zinc phthalocyanines have attracted increasing interests and attentions in the photodynamic therapy, non-linear optoelectronic devices, sensitive chemosensors, and photocatalysis for visible-light-driven degradation of organic dyes, thanks to their rich photochemical and photophysical properties including near-infrared emission, high singlet oxygen generation efficiency, and excellent photostability [24,25,26,27,28,29,30,31]. In the above-mentioned applications, a larger number of chemical protocols were utilized to synthesize soluble and non-aggregated zinc phthalocyanine complexes, mainly because of the fact that aggregated zinc phthalocyanines show attenuated or completely quenched optical properties [32,33,34,35]. Specifically, the reason for the quenched luminescence of zinc phthalocyanines was attributed to the formation of less emissive species including delocalized excimers or excitons upon aggregation. Meanwhile, photoluminescent properties for zinc phthalocyanine molecules have been hardly investigated in the solid state or in thin films.
In this work, we reported a series of dendritic zinc phthalocyanines showing both molecular emission and AIE-enabled aggregated or solid-state fluorescence, and their chemical structures, photophysical properties, intramolecular charge transfers, as well as morphology of self-assembling superstructures were fully characterized.
Experimental
Materials
4-nitrophthalonitrile, hydroquinone, 2, 6-naphthalenediol, 4, 4′-dihydroxybiphenyl, 4, 4′-dihydroxydiphenylmethane, and bisphenol A were obtained from Adamas Reagent Co. Ltd (Shanghai, China), while anhydrous potassium carbonate, zinc chloride, ammonium molybdate, dimethyl sulfoxide, methanol, acetone, ethanol, dimethylacetamide, and N, N-dimethylformamide were received from Kelong Reagent Co. Ltd (Chengdu, China). The polymethyl methacrylate (PMMA) was obtained from Titan Scientific Co. Ltd (Shanghai, China). All the chemical materials were used as received without any further purification.
Measurements
Fourier transform infrared spectra of the prepared compounds were characterized with Shimadzu 8400S spectrometer. NMR spectra were obtained with a Bruker AV II-400 spectrometer. The 1H-NMR (400 MHz) chemical shifts were measured relative to DMSO-d6 (H: δ = 2.50 ppm) as the internal references. Elemental analyses were performed by a Vario EL microelemental analyzer. The weight average molecular weight (M w), number average molecular weight (M n), and polydispersity index (M w/M n) were measured with gel permeation chromatography (PL-GPC220, Agilent). The melting temperature (T m) of the synthesized compounds was tested with a TA Instruments DSC-Q100 at a heating rate of 20 °C min−1. Thermal gravimetric analysis (TGA) of the prepared samples was conducted with a TA Instrument TGA-Q50 at a heating rate of 20 °C min−1. The UV–Vis absorption spectra for dendritic zinc phthalocyanines in DMF solution and DMF/H2O mixed solvent were recorded using a Persee TU 1901 UV–Vis spectrophotometer. The fluorescence excitation and emission spectra of dendritic zinc phthalocyanines in DMF solvent, in DMF/H2O mixed solution, as well as in the solid state were obtained using a fluorescence spectrophotometer (F-4600, Hitachi). The scanning electron microscope (JSM-6490LV, JEOL) was utilized to obtain the self-assembling microstructure of dendritic zinc phthalocyanines in DMF/H2O (50/50 vol%) mixed solution and surface morphology of the prepared polymer composite film. The fluorescence images were recorded with a fluorescent microscope (DP80, Olympus). The photos of sample vials under UV light were captured using a DSLR camera (D7000, Nikon).
Synthesis
The synthetic route and chemical structures of the compounds 2A–2E and dendritic zinc phthalocyanines 3A–3E are shown in Scheme 1. The specific procedure was demonstrated in the following.
Synthesis of compounds 2A–2E
The mixture containing compound 1 (2.9 mmol), 4-nitrophthalonitrile (1.00 g, 5.8 mmol), and anhydrous potassium carbonate (K2CO3) (0.90 g, 6.5 mmol) in 15 mL dimethyl sulfoxide (DMSO) solution was stirred at room temperature for 24 h under nitrogen atmosphere. The thermal transition and chemical structures of the compounds were characterized using DSC, FTIR, and 1H-NMR spectra as well as elemental analyses. The DSC curves of the synthesized compounds 2A–2E are shown in Fig. S1 in Electronic Supplementary Information (ESI).
Compound 2A
The reaction mixture was poured into H2O, and the precipitate was washed with H2O for three times and hot methanol for two times. The pure product was dried under vacuum for 12 h to obtain white powder. Yield: 0.75 g, 71.4%. T m: 260.4 °C. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.14 (d, J = 8.8 Hz, 2H, –C6H3), 7.88 (d, J = 2 Hz, 2H, –C6H3), 7.53–7.51 (m, 2H, –C6H3), 7.34 (s, 4H, –C6H4). IR (KBr, cm−1): 3108, 3077, 3049, 2233, 1596, 1562, 1498, 1482, 1285, 1248, 1191, 1091, 1016. Anal.Calcd. (%) for C22H10N4O2: C 72.92; H 2.78; N 15.46; found: C 72.92; H 2.82; N 15.35.
Compound 2B
The reaction mixture was poured into H2O, and the precipitate was washed with H2O for three times, acetone for two times, and hot methanol for two times. The pure product was dried under vacuum for 12 h to obtain white powder. Yield: 0.93 g, 77.4%. T m: 266.9 °C. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.14 (d, J = 8.8 Hz, 2H, –C6H3), 8.07 (d, J = 8.8 Hz, 2H, –C10H6), 7.90 (s, 2H, –C6H3), 7.82 (s, 2H, –C6H3), 7.50–7.44 (t, 4H, –C10H6). IR (KBr, cm−1): 3112, 3078, 3044, 2234, 1592, 1566, 1492, 1414, 1380, 1308, 1282, 1256, 1208, 1145, 1109, 1085. Anal.Calcd. (%) for C26H12N4O2: C 75.72; H 2.93; N 13.59; found: C 75.80; H 2.90; N 13.53.
Compound 2C
The reaction mixture was poured into H2O, and the precipitate was washed with H2O for three times, acetone for two times, and hot methanol for two times. The pure product was dried under vacuum for 12 h to obtain white powder. Yield: 0.98 g, 76.8%. T m: 234.6 °C. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.13 (d, J = 8.8 Hz, 2H, –C6H3), 7.87 (d, J = 2 Hz, 2H, –C6H3), 7.82 (d, J = 8.4 Hz, 4H, –C12H8), 7.47–7.45 (m, 2H, –C6H3), 7.31 (d, J = 8.4 Hz, 4H, –C12H8). IR (KBr, cm−1): 3109, 3075, 3039, 2233, 1592, 1489, 1417, 1384, 1317, 1285, 1252, 1205, 1159, 1090, 1007. Anal.Calcd. (%) for C28H14N4O2: C 76.70; H 3.22; N 12.78; found: C 77.05; H 3.18; N 12.72.
Compound 2D
The reaction mixture was poured into H2O, and the precipitate was washed with H2O for three times, alcohol for two times, and hot methanol for two times. The pure product was dried under vacuum for 12 h to obtain white powder. Yield: 1.15 g, 87.7%. T m: 196.5 °C. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.08 (d, J = 8.8 Hz, 2H, –C6H3), 7.78 (s, 2H, –C6H3), 7.37 (t, 6H, Ar–H), 7.14 (d, J = 8.0 Hz, 4H, –C6H4), 4.05 (s, 2H, –CH2–). IR (KBr, cm−1): 3080, 3040, 2927, 2232, 1592, 1565, 1501, 1484, 1418, 1286, 1278, 1250, 1202, 1167, 1094, 1017. Anal.Calcd. (%) for C29H16N4O2: C 76.98; H 3.56; N 12.38; found: C 76.90; H 3.60; N 12.52.
Compound 2E
The reaction mixture was poured into H2O, and the precipitate was washed with H2O for three times, alcohol for two times, and hot methanol for two times. The pure product was dried under vacuum for 12 h to obtain white powder. Yield: 1.22 g, 87.6%. T m: 196.5 °C. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.10 (d, J = 8.8 Hz, 2H, –C6H3), 7.80 (s, 2H, –C6H3), 7.37 (d, J = 8.4 Hz, 6H, Ar–H), 7.13 (d, J = 8.4 Hz, 4H, –C6H4), 1.69 (s, 6H, –CH3). IR (KBr, cm−1): 3108, 3075, 3046, 2975, 2233, 1590, 1561, 1503, 1489, 1421, 1305, 1289, 1256, 1211, 1178, 1083, 1016. Anal.Calcd. (%) for C31H20N4O2: C 77.49; H 4.20; N 11.66; found: C 77.60; H 4.15; N 11.59.
Synthesis of dendritic zinc phthalocyanines 3A–3E
A mixture of compound 2 (2 mmol), zinc chloride (0.068 g, 0.5 mmol), and ammonium molybdate (5 mg) in 20 mL of dimethylacetamide (DMAC) was heated at reflux under stirring. After the reaction liquid color turned into light-green, the mixture was kept reflux for 4.0 h. Next, the reaction mixture was poured into H2O, and the precipitate was washed with H2O for three times, hot methanol for two times, and dried under vacuum for 12 h. The chemical structures of the dendritic zinc phthalocyanines were characterized using UV–Vis, GPC, FTIR, and 1H-NMR spectra. The 1H-NMR spectra of dendritic zinc phthalocyanines 3A–3E are displayed in Fig. S10 in ESI.
Dendritic zinc phthalocyanine 3A
light-green powder, yield: 0.27 g. 37.3%. 1H–NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.13 (d, J = 8.8 Hz, Pc–H), 7.88–7.83 (m, Pc–H), 7.41 (d, J = 7.6 Hz, Pc–H), 7.34–7.27 (m, –C6H4). IR (KBr, cm−1): 2233, 1766, 1720, 1610, 1499, 1476, 1447, 1365, 1309, 1268, 1229, 1190, 1089, 1040. GPC (relative to polystyrene standards): M w = 1801, M n = 1712, M w/M n = 1.05. UV–Vis (DMF, nm): λ max1 = 610, λ max2 = 679.
Dendritic zinc phthalocyanine 3B
light-green powder, yield: 0.25 g, 30.3%. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.14 (d, J = 8.8 Hz, Pc-H), 8.07 (d, J = 8.4 Hz, –C10H6), 7.90 (d, J = 8.0 Hz, Pc–H), 7.87–7.77 (m, Pc–H), 7.50–7.42 (m, –C10H6). IR (KBr, cm−1): 2234, 1769, 1721, 1595, 1484, 1444, 1380, 1309, 1278, 1146, 1112, 1042. GPC (relative to polystyrene standards): M w = 1989, M n = 1872, M w/M n = 1.06. UV–Vis (DMF, nm): λ max1 = 611, λ max2 = 680.
Dendritic zinc phthalocyanine 3C
light-green powder, yield: 0.32 g, 36.5%. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.13 (d, J = 8.8 Hz, Pc–H), 7.86 (s, Pc–H), 7.82 (d, J = 7.6 Hz, –C12H8), 7.47–7.41 (m, Pc–H), 7.30 (d, J = 8.8 Hz, –C12H8). IR (KBr, cm−1): 2232, 1769, 1720, 1593, 1486, 1361, 1312, 1279, 1247, 1207, 1166, 1089, 1041, 1008. GPC (relative to polystyrene standards): M w = 2122, M n = 2096, M w/M n = 1.01. UV–Vis (DMF, nm): λ max1 = 610, λ max2 = 678.
Dendritic zinc phthalocyanine 3D
light-green powder, yield: 0.20 g, 22.1%. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.08 (d, J = 8.0 Hz, Pc–H), 7.80 (d, J = 9.6 Hz, Pc–H), 7.37–7.32 (m, Ar–H), 7.19–7.12 (m, –C6H4), 4.03 (s, –CH2–). IR (KBr, cm−1): 2232, 1767, 1719, 1652, 1598, 1561, 1502, 1478, 1441, 1363, 1312, 1276, 1234, 1203, 1167, 1092, 1041. GPC (relative to polystyrene standards): M w = 2347, M n = 2306, M w/M n = 1.02. UV–Vis (DMF, nm): λ max1 = 611, λ max2 = 680.
Dendritic zinc phthalocyanine 3E
light-green powder, yield: 0.15 g, 15.6%. 1H-NMR [DMSO-d6, 400 MHz, δ (ppm)]: 8.09 (d, J = 8.6 Hz, Pc–H), 7.79 (s, Pc–H), 7.35 (s, Ar–H), 7.11 (s, –C6H4), 1.69 (s, –CH3). IR (KBr, cm−1): 2233, 1770, 1723, 1652, 1560, 1504, 1480, 1427, 1385, 1310, 1276, 1234, 1173, 1084, 1016. GPC (relative to polystyrene standards): M w = 2633, M n = 2415, M w/M n = 1.09. UV–Vis (DMF, nm): λ max1 = 610, λ max2 = 681.
Preparation of zinc phthalocyanine–polymer composite film
The polymethyl methacrylate (125 mg) was dissolved in THF solution (5.0 mL) by stirring at room temperature for 12 h. Then the DMF solution (1.0 mL) of dendritic zinc phthalocyanine 3A at a concentration of 1.0 and 2.0 mg mL−1 was added into the PMMA/THF mixture (5.0 mL), respectively. Finally, zinc phthalocyanine–polymer composite films were prepared using a spinning coater (KW-4A, Chemat Technology) at a rotation rate of 4000 rpm for 30 s and dried under vacuum at 30 °C for 12 h.
Results and discussion
Synthesis
As shown in Scheme 1, the compounds 2A–2E were successfully synthesized via a nucleophilic substitution reaction of 4-nitrophthalonitrile with compound 1A–1E in the presence of anhydrous potassium carbonate as the alkaline catalyst in DMSO solvent at room temperature, respectively. The cycloaddition reaction of compounds 2A–2E with zinc chloride using the ammonium molybdate as the catalyst in DMAC solution was utilized to prepare dendritic zinc phthalocyanines 3A–3E. All the studied zinc phthalocyanines could be obtained successfully through two-step synthesis, and the chemical structures of the synthesized dendritic zinc phthalocyanines were fully characterized with ultraviolet–visible absorption, infrared spectroscopy, and hydrogen nuclear magnetic resonance spectroscopy as well as gel permeation chromatography.
Electronic absorption and fluorescence spectra
The electronic absorption of dendritic zinc phthalocyanines 3A–3E exhibited representative Q-band absorption in the visible light region at around 600–700 nm in DMF solution, and the Q-band absorption spectral data of dendritic zinc phthalocyanines 3A–3E are summarized in Table 1. The UV–Vis optical spectroscopy of dendritic zinc phthalocyanines in DMF solution is shown in Fig. S2 (using dendritic zinc phthalocyanine 3A as an example). The dendritic zinc phthalocyanine 3A showed an intense UV absorption peak at 275 nm (B-band) along with a characteristic absorbance peak in the range of 600–700 nm (see inset of Fig. S2). While the B-band around 200–400 nm is attributed to the
a2u–eg transition, the typical Q-band corresponds to the π–π* (a1u–eg) transition of phthalocyanine rings from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) [36, 37].
Aggregation behavior of metallized phthalocyanine complexes was usually investigated because of the fact that photoactive properties of these complexes could be controlled through the change of molecular state from single monomer to dimer and highly ordered supramolecules. In this work, the aggregation behavior of dendritic zinc phthalocyanines at different concentrations in DMF solution was examined using dendritic zinc phthalocyanine 3A as an example. As shown in Fig. 1, when the concentration increased from 0.05 to 0.60 mg mL−1, dendritic zinc phthalocyanine 3A showed proportionally increased Q-band absorption at 600–700 nm region without any peak shift or distortion, meanwhile the linear Lambert–Beer law was observed from Q-band absorption intensity at 679 nm versus concentration of 3A solution (see inset of Fig. 1), which demonstrated that dendritic zinc phthalocyanine aggregate was not formed at concentrations ranging from 0.05 to 0.60 mg mL−1.
The fluorescence excitation and emission spectra of dendritic zinc phthalocyanines were recorded in DMF solution as shown in Fig. S3 (using dendritic zinc phthalocyanine 3A as an example). It was observed that three fluorescent excitation peaks were detected at 365, 616, and 682 nm for dendritic zinc phthalocyanine 3A through monitoring the fluorescent emission at 694 nm. The dendritic zinc phthalocyanine 3A exhibited a sharp red emission peak at ~694 nm along with a broad blue emission at approximately 439 nm when the sample was excited at 365 nm, and it was clear that only single fluorescent emission peak at 694 nm was found under excitation at 616 nm and 682 nm. And the excitation and emission spectral data of dendritic zinc phthalocyanines 3B–3E are fully summarized in Table 1. All the samples in DMF solution exhibited dual-emissive spectra at ~440 and ~695 nm as observed in Table 1, and the stokes shift of all dendritic zinc phthalocyanines was around 330 nm.
Fluorescence properties in the molecular state
The fluorescent properties of zinc phthalocyanines in the molecular state were dependent on the nature of the solution, the concentration, and the substituents. The dendritic zinc phthalocyanines 3A–3E possessed good solubility in most organic solvents including dimethylformamide, dimethylacetamide, dimethylsulfoxide, and pyridine as well as THF. Specifically, dendritic zinc phthalocyanine 3E showed largest solubility in DMF when compared to other dendritic zinc phthalocyanines, which should be attributed to the more sterically demanding configuration presented in dendritic zinc phthalocyanine 3E. The fluorescence emission spectra of dendritic zinc phthalocyanines at concentrations ranging from minimum concentration (0.02 mg mL−1) to maximum concentration (6.00 mg mL−1) are shown in Fig. 2 (using dendritic zinc phthalocyanine 3A as an example). As seen in Fig. 2a, when the concentration increased, the red emission peak red-shifted ranging from 692 to 715 nm and blue band emission peak also red-shifted. This demonstrated that the concentration of dendritic zinc phthalocyanine 3A in DMF solution plays an important role for the location of fluorescent emission peak resulting from the formation of zinc phthalocyanine dimers. Figure 2b shows the plot of red emission intensity versus concentration; the maximum emission intensity was clearly observed when the concentration of dendritic zinc phthalocyanine 3A was at ~0.40 mg mL−1 as displayed the image under UV light illumination in inset of Fig. 2b. The decreased red fluorescent emission intensity of dendritic zinc phthalocyanine 3A at maximum concentration in DMF solution was clearly observed, and the photoluminescent intensity of the concentrated solution of dendritic zinc phthalocyanine 3A was weak when compared to that of the dilute solvent, which implied that dendritic zinc phthalocyanine molecules under the condition of high concentration in DMF solution tend to form H-type (face to face) aggregation rather than J-type (head to tail) aggregation due to the combination of strong intermolecular π–π interactions and metal–ligand coordination bonding [38,39,40,41].
The fluorescence emission spectra of dendritic zinc phthalocyanine 3A in DMF solution under excitation at 365 nm at different concentrations: 0.02, 0.05, 0.10, 0.20, 0.40, 0.60, 1.00, 2.00, 3.00, 6.00 mg mL−1 (a) and plot of red emission intensity versus concentration (b). Inset Image of sample under UV irradiation at 365 nm in DMF solution using a cuvette
Fluorescence properties in the aggregated state
Similar to the conventional fluorophores, the fluorescence emission of dendritic zinc phthalocyanines 3D and 3E dispersed in DMF/H2O mixed solution was obviously quenched compared to that recorded from DMF solution as displayed in Fig. S4. More specifically, the DMF solution of 3D and 3E was highly fluorescent with dual-emissive peaks at ~440 and 695 nm, and their emission intensity was obviously reduced when water was introduced into DMF solvent. The intensity of red fluorescence was so poor when water fraction was up to >30 vol% and blue emission was also attenuated, suggesting that abundant molecules of dendritic zinc phthalocyanines become aggregated. These results confirmed that the fluorescent emission of dendritic zinc phthalocyanines 3D and 3E was basically determined by the typical ACQ effect. Furthermore, the electronic absorption was used to probe the aggregation formation in DMF/H2O mixed solution. The Q-band absorption at around 600–700 nm was red-shifted and became broader as shown in Fig. S4, which confirmed that zinc phthalocyanines might experience intense π–π interactions, leading to the formation of detrimental species that strongly quenched fluorescence [42].
Quite interestingly, we discovered that the fluorescent emission of dendritic zinc phthalocyanines 3A, 3B, and 3C in DMF/H2O solution can be modulated between ACQ and AIE-dominated framework, depending on the water fractions in mixed solution. As shown in Fig. 3, the intense sharp red emission at ~695 nm along with broad blue emission was obviously observed for dendritic zinc phthalocyanines 3A, 3B, and 3C in pure DMF solvent, and the similar behavior was displayed for dendritic zinc phthalocyanines 3D and 3E (see Fig. S4). When the zinc phthalocyanines were dispersed in the DMF/H2O mixed solvents where H2O volume ratio was less than 30%, their red-emitting peak intensity was quenched as increasing of water quantity, which indicated the fluorescence of dendritic zinc phthalocyanines 3A, 3B, and 3C were mainly determined by ACQ effect in this range. However, as more than 30% water was added to induce zinc phthalocyanine aggregation formation (see red-shift and broaden UV–Vis spectra shown in Fig. S5), it was clear from Fig. 3 that the blue-emissive peak for all these samples in the aqueous mixtures gradually appeared. The enhanced blue-emissive intensity was recorded by increasing water content, and a highest broad blue-emissive signal was obtained in 50 vol% aqueous mixture. These spectra data demonstrated that dendritic zinc phthalocyanines 3A, 3B, and 3C were typical blue AIE fluorophores. It was believed that the restriction of intramolecular rotation and strong π–π interactions were main reasons for the observed AIE enhancement. Afterward, the intensity of broad blue emission was again quenched when water fraction was higher than 50%, indicating that the ACQ again became the dominant factor for fluorescence of random zinc phthalocyanine aggregates generated via the fast precipitation process. Based on the above results, the fluorescent emission of dendritic zinc phthalocyanines 3A, 3B, and 3C can be readily modulated from basically red emitting in molecular state to blue fluorescent in aggregated state.
The fluorescence emission spectra of dendritic zinc phthalocyanines 3A (a), 3B (b), and 3C (c) under excitation at 365 nm in DMF/H2O mixture with different H2O fractions and the plots of fluorescent emission intensity of dendritic zinc phthalocyanines 3A (d), 3B (e), and 3C (f) versus the water fraction in the DMF/H2O mixed solution
To well understand the kinetics of the AIE effect for dendritic zinc phthalocyanines 3A–3C, influence of the aggregating time on their fluorescence emission in DMF/H2O (50/50 vol%) mixed solution was investigated. Equal volume of water was immediately added into the DMF solvent of dendritic zinc phthalocyanines to obtain the DMF/H2O mixture. Next, the emission spectrum of the mixture was recorded using a fluorescence spectrophotometer at a specific time interval. As shown in Fig. 4a, the blue emission intensity of dendritic zinc phthalocyanine 3C was enhanced drastically for the first 30 min, and reached a maximum emissive value thereafter. However, the maximum value of fluorescence emission for dendritic zinc phthalocyanine 3A was obtained within 2 min as displayed in Fig. 4b. This demonstrated that intramolecular rotation of dendritic zinc phthalocyanine 3A was restricted more rapidly when compared to 3C, as the biphenyl groups of dendritic zinc phthalocyanine 3C were larger than others and more time was required to enhance the restricted intramolecular rotation effects.
Furthermore, the surface morphology of zinc phthalocyanine supramolecular structures formed during aggregation process was characterized with SEM. Taking dendritic zinc phthalocyanine 3A as an example, the well-organized self-assembled superstructures with different morphologies could be successfully formed. Specifically, equal volume of water was rapidly added into DMF solution of 3A, and the resulted mixture of phthalocyanine aggregates was kept at room temperature for 60 s, during which the two-dimensional (2D) microstructures were obtained as displayed in Fig. 5a. From the high-magnification SEM image (see Fig. 5b), it was clear that the 2D microstructure was a well-designed leaf-like supramolecular microstructure. We assumed that this leaf-like structure was developed via the following steps: a rigid rod-like building block was initially formed via self-assembly of zinc phthalocyanines, which served as the “seeds” for further growth of more rod-like molecules. Thus, it was assumed that the driven force for the rod-like microstructure formation was the combination of metal–ligand coordination bonding, intermolecular cyano interactions, and strong intermolecular π–π interactions [43]. More interestingly, when the aggregating time was prolonged to 120 s, the self-assembled three-dimensional (3D) microspheres could be successfully formed as observed in the low-magnification SEM image (see Fig. 5c). Furthermore, as shown in high-magnification SEM image of Fig. 5d, the self-assembled supramolecular aggregation was a well-organized 3D microsphere which was composed of abundant assembling rod-like building blocks. Based on the obtained result from the fluorescence spectra as seen in Fig. 4, it could be concluded that the 3D microsphere was highly photoluminescent compared to the 2D leaf-like microstructure.
Dependence of self-assembled supramolecular structures for dendritic zinc phthalocyanine 3A to aggregating time in DMF/H2O (50/50 vol%) mixture: low-magnification SEM image (a) and high-magnification SEM image (b) of supramolecular structures at 60 s; low-magnification SEM image (c) and high-magnification SEM image (d) of supramolecular structures at 120 s
Fluorescence properties in the solid state
It is well known that conventional metallized phthalocyanines in the solid state exhibit non-photoluminescence properties mainly due to delocalized excimers or excitons leading to enhanced non-radiative deactivation [44]. However, dendritic zinc phthalocyanines 3A, 3B, and 3C in this work showed attractive photoactive properties as displayed in Fig. S6. Two peaks at ~280 and 365 nm were detected from excitation spectra of dendritic zinc phthalocyanines 3A, 3B, and 3C in the solid state, and the highest fluorescence emission intensity of dendritic zinc phthalocyanines could be obtained under excitation at 365 nm. As shown in Fig. 6, dendritic zinc phthalocyanine 3C exhibited the maximum fluorescent emission intensity, and the emitting intensity of dendritic zinc phthalocyanine 3C was nearly two-fold larger than that of dendritic zinc phthalocyanines 3A and 3B. The enhanced emission intensity of dendritic zinc phthalocyanine 3C was likely owing to the biphenyl group presented in dendritic zinc phthalocyanine 3C. The naphthalene-conjugated structure leads to the red-shifted emission wavelength of dendritic zinc phthalocyanine 3B (c. 487 nm) in the solid state when compared to that of dendritic zinc phthalocyanines 3A (465 nm) and 3C (468 nm). Meanwhile, it can be obviously observed that dendritic zinc phthalocyanines containing diphenylmethane or bisphenol A units (3D and 3E) in the solid state showed quite weak fluorescence emission in the visible light region, which might be attributed to the flexible structure of the diphenylmethane and bisphenol A units resulting in the free intramolecular rotation effects. The color of dendritic zinc phthalocyanines 3A–3D was light-green, while 3E exhibited green under visible light illumination as shown in Fig. 6b. Under UV lamp excitation (365 nm), dendritic zinc phthalocyanine 3A was authentic blue and green color was detected from 3B. Moreover, dendritic zinc phthalocyanine 3C showed highest luminescence which could be confirmed by the fluorescence spectrum.
It has been believed that the solid-state fluorescence properties of dendritic zinc phthalocyanines 3A–3E have a close relationship with the chemical structure of their corresponding precursor compounds 2A–2E according to our best knowledge. Therefore, the density functional theory (DFT) calculations were carried out at the hybrid B3LYP level using the split valence 6-31G basis set to simulate the optimized molecular configuration and charge transfer process. The data obtained from DFT calculations including HOMO energy, LUMO energy, and HOMO–LUMO energy gap are fully summarized in Table S1. The optimized molecular configuration and the electron density distribution of the HOMO and LUMO levels for compounds 2A–2E are shown in Fig. 7. It was clearly observed that molecular structures of compounds 2A, 2B, and 2C showed better planarity in comparison to that of compounds 2D and 2E resulting in the easier charge transfer from the HOMO to the LUMO, and the charge transfer of compound 2C was enhanced compared to that of compounds 2A and 2B, which should be due to the presence of longer conjugation bridge of biphenyl. The enhanced charge transfer of compound 2C would normally contribute to an easier electron transition, which in turn leads to its increased luminescence. Meanwhile, the lowest HOMO–LUMO energy gap of 4.287 eV was recorded for compound 2B as shown in Table S1, which was well correlated with the largest fluorescent emission wavelength of dendritic zinc phthalocyanine 3B. Interestingly, compounds 2D and 2E displayed totally different electron density distribution compared with those of compounds 2A, 2B, and 2C. More specifically, the electrons, uniformly dispersed in the overall molecule of 2D at ground state, were obviously transferred to the phthalonitrile moiety with strong electron-withdrawing ability upon photoexcitation, and the charge transfer process from HOMO to LUMO was further enhanced for 2E. The nearly “total charge transfer” for 2D and 2E should be attributed to the presence of electron-donating groups (methylene and propyl), which would promote the photo-induced electron transfer process that strongly quenches their luminescence.
Thermal stability of metallized phthalocyanines is important for their practical applications; thus, the thermal properties of dendritic zinc phthalocyanines 3A–3E were evaluated by the TGA analysis as shown in Fig. S7, and the detail data from the TGA curves are listed in Table 2. All dendritic zinc phthalocyanines 3A–3E exhibited good thermal stability, and the temperatures corresponding to 5wt% weight loss (T 5%) are higher than 350 °C. The maximum value of T 5% was detected from dendritic zinc phthalocyanine 3C (396.61 °C), which would be attributed to molecular structure of compound 2C. However, the T 5% value was lowest for dendritic zinc phthalocyanine 3E (357.62 °C) mainly owing to the destruction of rigid construction and conjugated degree by propyl group.
Fluorescent images of zinc phthalocyanine–polymer composite film
As discussed previously, the luminescent properties of dendritic zinc phthalocyanines 3A–3C can be well modulated from molecular state to aggregated state and solid state. Therefore, the experimental investigations of polymer composite film with different concentrations of zinc phthalocyanines are of great interests. In this work, PMMA solution containing different concentrations of dendritic zinc phthalocyanines (using dendritic zinc phthalocyanine 3A as an example) was spin coated to fabricated flexible fluorescent film. As displayed in Fig. S8, the small particles were clearly observed and well dispersed from low-concentration sample, while vast rod-like structures were found and local regions have abundant rod-like structures in high-concentration image. It was believed that the small particle and rod-like structure were attributed to the nanoassembles of zinc phthalocyanine molecules. For polymer composite film containing lower concentration of 3A, the weak blue-emitting fluorescence and intense red fluorescence were detected from fluorescent microscope images (see Fig. 8a, b), while for polymer composite film doping with higher content of 3A, the intense blue fluorescence along with quenched red emission was observed due to the presence of zinc phthalocyanine aggregates (see Fig. 8c, d). These results demonstrated that the luminescent behavior of zinc phthalocyanine–polymer composite film could be readily modulated by controlling the doping concentration of zinc phthalocyanines, which was similar to the emissive spectra of dendritic zinc phthalocyanines in DMF/H2O mixed solution.
The surface morphology of zinc phthalocyanine–polymer composite film has been investigated utilizing a scanning electron microscope. It can be seen that the polymer composite film displayed outstanding transmittance because of their loose network structures. Meanwhile, the luminescence properties of dendritic zinc phthalocyanines were not almost influenced after introducing the PMMA into THF/DMF mixed solvent (see Fig. S9). As shown in Fig. 9a, b, zinc phthalocyanine molecular aggregates were not found for the polymer composite film with a low concentration of dendritic zinc phthalocyanines. On the contrary, abundant assembling rod-like microstructures had been detected from the polymer composite film with a high concentration of dendritic zinc phthalocyanines as visualized in Fig. 9c. It was clear that a rod-like microstructure was embedded in the loose network structure of PMMA observed in Fig. 9d. The results assumed that the PMMA plays an important character about the formation of dendritic zinc phthalocyanine aggregation.
Conclusions
In conclusion, we designed and synthesized a new family of fluorophores based on dendritic zinc phthalocyanines that can emit strongly in molecular, aggregated, solid states as well as in polymer composite films. Specifically, dendritic zinc phthalocyanines 3A–3C exhibited enhanced blue-emitting luminescence in comparison to typical ACQ characteristics of dendritic zinc phthalocyanines 3D–3E upon their aggregation. More interestingly, novel dendritic zinc phthalocyanines 3A–3C showed an interesting change from highly NIR emitting in molecular state to strongly blue fluorescence in aggregated state. Aggregating time-dependent fluorescence emission and self-assembling supramolecular microstructures in 50 vol% aqueous mixture were fully investigated, and a well-organized self-assembled aggregation including 3D microsphere and 2D leaf-like microstructure for dendritic zinc phthalocyanine 3A could be successfully obtained. Meanwhile, these dendritic zinc phthalocyanines 3A–3C displayed solid-state luminescence, and their solid-state fluorescence was rationalized in terms of molecular configuration and charge transfer as clarified by the DFT calculations. Moreover, dendritic zinc phthalocyanine-doped PMMA polymer composite films were prepared via a simple approach, and their luminescent behaviors were systematically explored. Based on the above results, we believed that dendritic zinc phthalocyanines 3A–3C could find potential applications in high-tech fields of biological imaging agents, fluorescent chemosensor, as well as flexible optoelectronic devices.
References
Mandal K, Jana D, Ghorai BK, Jana NR (2016) Fluorescent imaging probe from nanoparticle made of AIE molecule. J Phys Chem C 120:5196–5206
Yin W, Zhu H, Wang R (2014) A sensitive and selective fluorescence probe based fluorescein for detection of hypochlorous acid and its application for biological imaging. Dyes Pigments 107:127–132
Zhu X, Lin Q, Chen P, Fu YP, Zhang YM, Wei TB (2016) A novel pH sensor which could respond to multi-scale pH changes via different fluorescence emissions. New J Chem 40:4562–4565
Shi H, Xin D, Bai SD et al (2016) The synthesis, crystal structures, aggregation-induced emission and electroluminescence properties of two novel green-yellow emitters based on carbazole-substituted diphenylethene and dimesitylboron. Org Electron 33:78–87
Thomas SW, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:1339–1386
Yuan WZ, Lu P, Chen S et al (2010) Changing the behavior of chromophores from aggregation-caused quenching to aggregation-induced emission: development of highly efficient light emitters in the solid state. Adv Mater 22:2159–2163
Song QH, Wu QQ, Liu CH, Du XJ, Guo QX (2013) A novel fluorescent probe for selective detection of thiols in acidic solutions and labeling of acidic organelles in live cells. J Mater Chem B 1:438–442
Lee IH, Song W, Lee JY (2016) Aggregation-induced emission type thermally activated delayed fluorescent materials for high efficiency in non-doped organic light-emitting diodes. Org Electron 29:22–26
Lei Y, Li H, Gao W et al (2014) Highly sensitive conjugated polymer fluorescent sensors based on benzochalcogendiazole for nickel ions in real-time detection. J Mater Chem C 2:7402–7410
Wang Y, Liu W, Bu L et al (2013) Reversible piezochromic luminescence of 9,10-bis[(N-alkylcarbazol-3-yl)vinyl]anthracenes and the dependence on N-alkyl chain length. J Mater Chem C 1:856–862
Liu Y, Tao X, Wang F et al (2008) Aggregation-induced emissions of fluorenonearylamine derivatives: a new kind of materials for nondoped red organic light-emitting diodes. J Phys Chem C 112:3975–3981
Zhao Z, Chen S, Lam JWY et al (2010) steric hindrance, electronic communication, and energy transfer in the photo—and electroluminescence processes of aggregation-induced emission luminogens. J Phys Chem C 114:7963–7972
Luo J, Xie Z, Lam JWY et al (2001) Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun 18:1740–1741
Hong Y, Lam JWY, Tang BZ (2009) Aggregation-induced emission: phenomenon, mechanism and applications. Chem Commun 29:4332–4353
Hong Y, Lam JWY, Tang BZ (2011) Aggregation-induced emission. Chem Soc Rev 40:5361–5388
Zhao N, Lam JWY, Sung HHY et al (2014) Effect of the counterion on light emission: a displacement strategy to change the emission behaviour from aggregation-caused quenching to aggregation-induced emission and to construct sensitive fluorescent sensors for Hg2+ detection. Chem Eur J 20:133–138
Kwok RTK, Leung CWT, Lam JWY, Tang BZ (2015) Biosensing by luminogens with aggregation-induced emission characteristics. Chem Soc Rev 44:4228–4238
Zhang Y, Li J, Tang BZ, Wong KS (2014) Aggregation enhancement on two-photon optical properties of AIE-Active D-TPE-A molecules. J Phys Chem C 118:26981–26986
Zheng J, Huang F, Li Y et al (2015) The aggregation-induced emission enhancement properties of BF2 complex isatin-phenylhydrazone: synthesis and fluorescence characteristics. Dyes Pigments 113:502–509
Botta C, Benedini S, Carlucci L et al (2016) Polymorphism-dependent aggregation induced emission of a push-pull dye and its multi-stimuli responsive behavior. J Mater Chem C 4:2979–2989
Xu F, Wang H, Du X et al (2016) Structure, property and mechanism study of fluorenone-based AIE dyes. Dyes Pigments 129:121–128
Yin G, Ma Y, Xiong Y, Cao X, Li Y, Chen L (2016) Enhanced AIE and different stimuli-responses in red fluorescent (1,3-dimethyl)barbituric acid-functionalized anthracenes. J Mater Chem C 4:751–757
Zhang Y, Li H, Zhang G et al (2016) Aggregation-induced emission enhancement and mechanofluorochromic properties of [small alpha]-cyanostilbene functionalized tetraphenyl imidazole derivatives. J Mater Chem C 4:2971–2978
Nyokong T (2007) Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coord Chem Rev 251:1707–1722
Gao Z, Tao X, Cui Y, Satoh T, Kakuchi T, Duan Q (2011) Synthesis of end-functionalized poly(N-isopropylacrylamide) with group of asymmetrical phthalocyanine via atom transfer radical polymerization and its photocatalytic oxidation of Rhodamine B. Polym Chem 2:2590–2596
Zheng BY, Zhang HP, Ke MR, Huang JD (2013) Synthesis and antifungal photodynamic activities of a series of novel zinc(II) phthalocyanines substituted with piperazinyl moieties. Dyes Pigments 99:185–191
Swain D, Singh R, Singh VK, Krishna NV, Giribabu L, Rao SV (2014) Sterically demanding zinc(II) phthalocyanines: synthesis, optical, electrochemical, nonlinear optical, excited state dynamics studies. J Mater Chem C 2:1711–1722
Anaya-Plaza E, Oliva MM, Kunzmann A et al (2015) Quaternized pyridyloxy phthalocyanines render aqueous electron-donor carbon nanotubes as unprecedented supramolecular materials for energy conversion. Adv Funct Mater 25:7418–7427
Venkatramaiah N, Rocha DMGC, Srikanth P, Almeida Paz FA, Tome JPC (2015) Synthesis and photophysical characterization of dimethylamine-derived Zn(II)phthalocyanines: exploring their potential as selective chemosensors for trinitrophenol. J Mater Chem C 3:1056–1067
Wang A, Gui L, Lu S, Zhou L, Zhou J, Wei S (2016) Tumor microenvironment-responsive charge reversal zinc phthalocyanines based on amino acids for photodynamic therapy. Dyes Pigments 126:239–250
Jia K, Pan L, Wang Z et al (2016) Morphology and photophysical properties of dual-emissive hyperbranched zinc phthalocyanines and their self-assembling superstructures. J Mater Sci 51:3191–3199. doi:10.1007/s10853-015-9630-8
Durmuş M, Yaman H, Göl C, Ahsen V, Nyokong T (2011) Water-soluble quaternized mercaptopyridine-substituted zinc-phthalocyanines: synthesis, photophysical, photochemical and bovine serum albumin binding properties. Dyes Pigments 91:153–163
Göl C, Durmuş M (2012) Investigation of photophysical, photochemical and bovine serum albumin binding properties of novel water-soluble zwitterionic zinc phthalocyanine complexes. Synth Met 162:605–613
Liu W, Jensen TJ, Fronczek FR, Hammer RP, Smith KM, Vicente MGH (2005) Synthesis and cellular studies of nonaggregated water-soluble phthalocyanines. J Med Chem 48:1033–1041
Dumoulin F, Durmuş M, Ahsen V, Nyokong T (2010) Synthetic pathways to water-soluble phthalocyanines and close analogs. Coord Chem Rev 254:2792–2847
Zhang M, Shao C, Guo Z et al (2011) Highly efficient decomposition of organic dye by aqueous-solid phase transfer and in situ photocatalysis using hierarchical copper phthalocyanine hollow spheres. ACS Appl Mater Interfaces 3:2573–2847
Nyokong T, Gasyna Z, Stillman MJ (1987) Analysis of the absorption and magnetic circular dichroism spectra of zinc phthalocyanine and the.pi.-cation-radical species [ZnPc(1-)].cntdot.+. Inorga Chem 26:1087–1095
Zhang C, Jing L, Lin S et al (2013) Helical self-assembly of optically active phthalocyanine derivatives: effect of zn-o coordination bond on morphology and handedness of nanostructures. ChemPhysChem 14:3827–3833
Zhang XF, Xi Q, Zhao J (2010) Fluorescent and triplet state photoactive J-type phthalocyanines nano assemblies: controlled formation and photo snesitizing properties. J Mater Chem 20:6726–6733
Morisue M, Morita T, Kuroda Y (2010) Ligand-assisted J-type aggregates of zinc porphyrin: anticooperative molecular organization in self-assembled bolaamphiphile. Org Biomol Chem 8:3457–3463
Adachi K, Watarai H (2005) Interfacial aggregation of thioether-substituted phthalocyaninatomagnesium(II)-palladium(II) complexes in the toluene/water system. J Mater Chem 15:4701–4710
Geng J, Zhu Z, Qin W et al (2014) Near-infrared fluorescence amplified organic nanoparticles with aggregation-induced emission characteristics for in vivo imaging. Nanoscale 6:939–945
Jang K, Kinyanjui JM, Hatchett DW, Lee DC (2009) Morphological Control of One-Dimensional Nanostructures of T-Shaped Asymmetric Bisphenazine. Chem Mater 21:2070–2076
Cornil J, Beljonne D, Calbert JP, Brédas JL (2001) Interchain interactions in organic π-conjugated materials: impact on electronic structure, optical response, and charge transport. Adv Mater 13:1053–1067
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (Project Nos. 51373028, 51403029), the Scientific Research Foundation for the Returned Overseas Chinese Scholars from State Education Ministry, South Wisdom Valley Innovative Research Team Program, and Ningbo Major Science and Technology Research Plan (2013B06011).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic Supplementary Information: The online version of this article contains supplementary material, which is available to authorized users.
Rights and permissions
About this article
Cite this article
Pan, L., Jia, K., Shou, H. et al. Unification of molecular NIR fluorescence and aggregation-induced blue emission via novel dendritic zinc phthalocyanines. J Mater Sci 52, 3402–3418 (2017). https://doi.org/10.1007/s10853-016-0628-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0628-7