Abstract
Ce3+-doped and Ce3+/Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphors were synthesized successfully via a traditional high-temperature solid-state reaction. The crystal structure, photoluminescent properties including temperature-dependent luminescence, and energy transfer of the as-prepared phosphors were investigated. The as-prepared Ca9Sr(PO4)6Cl2:Ce3+ phosphors exhibit a broad excitation band ranging from 220 to 385 nm and blue light-emitting band centered at 431 nm, which originate from the 4f–5d transitions of Ce3+ ion. The luminescent intensities of Tb3+ ions were dramatically enhanced by the introduction of Ce3+ in the Ca9Sr(PO4)6Cl2:Tb3+ phosphors because of the efficient energy transfer from Ce3+ to Tb3+ ions, generating tunable blue-green emission colors. The mechanism of energy transfer between Ce3+ and Tb3+ ions was demonstrated to be an electric dipole–quadrupole interaction. Moreover, the energy transfer efficiency was evaluated up to 75 % based on the analysis of the emission spectra. The temperature-dependent photoluminescence indicates that the as-prepared Ce3+/Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphors have excellent thermal stability. Our results suggest that the Ce3+-doped and Ce3+/Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphors have potential application for n-UV pumped WLEDs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
White light-emitting diodes (WLEDs) are considered as an ideal candidate for the replacement of conventional lighting resource because of their remarkable advantages such as long operation lifetime, high luminous efficiency, energy saving, and eco-friendliness [1–3]. Typically, the commercial WLEDs were assembled by a combination of a blue-emitting InGaN chip and a yellow-emitting Y3Al5O12:Ce3+ (YAG:Ce3+) phosphor. However, this type of device suffers from inevitable drawbacks including a low color rendering index (CRI < 75) and high correlated color temperature (CCT ≥ 4500 K) due to the lack of red component in the visible region [5–7]. Nowadays, in view of these problems, WLEDs fabricated by assembling ultraviolet (UV) or near-ultraviolet (n-UV) LED chips and tricolor (red, green, and blue) phosphors are expected to dominate the solid-state lighting (SSL) market in the future because the white light outputting from this type of WLEDs has controlled color temperature and exceptional color rendering index [1–4]. In addition, the performances of n-UV LEDs strongly depend on the luminescence performance of phosphors used. Therefore, the recent researches have focused on developing suitable tricolor phosphors with high luminescent efficiency and high stability for n-UV LEDs [4–6].
As we all know, Tb3+ ion is frequently used as an activator of green-emitting luminescent phosphors because it has a relatively simple structure of energy levels that consist of 7F J , 5D4, and 5D3 states. Usually, the 5D4–7F5 transition peaking at around 541 nm leads to the predominant green emission, which is a magnetic dipole transition with \( \Delta J\; = \;1 \) [9, 34]. However, the intensities of Tb3+ characteristic sharp lines at 488, 541, and 582 nm are very week and the spectral widths are quite narrow due to the strictly forbidden f–f absorption transitions as well. One of the efficient strategies to conquer the difficulty is to introduce Ce3+ as a sensitizer to transfer excitation energy to Tb3+ efficiently because there is larger spectral overlapping between the emission spectrum of Ce3+ ion and absorption spectrum of Tb3+ ion. Until now, several Ce3+/Tb3+ co-doped phosphors have been synthesized and investigated, such as Li6Lu(BO3)3:Ce3+, Tb3+; Y4Si2O7N2:Ce3+, Tb3+; Ca6Ba(PO4)4O:Ce3+, Tb3+; YBO3:Ce3+, Tb3+; KGdF4:Ce3+, Tb3+; GdPO4:Ce3+, Tb3+, and so on [3–20].
Materials belonging to the large apatite family described by the general formula of M10(TO4)6X2 (M = alkaline; X = monovalent anion, such as OH− Cl−, and F−; and TO4 3− = trivalent anions, such as PO4 3−, VO4 3−, or AsO4 3−) are widely used for n-UV LEDs owing to their many advantages including high chemical and physical stability, low cost, and excellent weather resistance [21–25]. A wide range of cationic and anionic substitutions are possible to create many modified apatite structures with improved luminescent performance [25]. Especially, Ca10(PO4)6Cl2 is a good host lattice for luminescence. Recent investigation was conducted on Ca10(PO4)6Cl2:Eu3+; Ca10(PO4)6Cl2:Eu2+ [21, 23]. To the best of our knowledge, there is no report on the luminescence of Ce3+-doped and Ce3+/Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphors yet. Herein, we prepared Ce3+-doped and Ce3+/Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphors via a high-temperature solid-state reaction method. The crystal structure, photoluminescent (PL) properties, color chromaticity, energy transfer, and thermal quenching of the as-prepared samples were investigated. The results show that Ce3+-doped and Ce3+/Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphors could be a potential blue-green tunable phosphor for n-UV pumped WLEDs.
Experimental section
Synthesis
Ca9Sr(PO4)6Cl2:x%Ce3+ (\( 1\; \le \;x\; \le \;7 \)) and Ca9Sr(PO4)6Cl2:5%Ce3+, y%Tb3+ (\( 1\; \le \;y\; \le \;7 \)) phosphors were prepared by a solid-state reaction from a stoichiometric mixture of CaCO3, SrCl2, (NH4)2HPO4, CeO2, and Tb4O7. The weighed raw chemicals were ground thoroughly in an agate mortar for 2.5 h, and then the obtained mixture was sintered in alumina crucibles at 1400 °C for 5 h under CO reducing atmosphere to produce the final sample. Finally, the prepared samples were cooled to room temperature and reground for further measurements.
Characterizations
To investigate the crystal phases of the as-prepared powder, X-ray diffractions (XRD) were performed in the range of \( 10\; \le \;2\theta \; \le \;70^{ \circ } \) with the step of 0.02o using a Shimadzu-6000 X-ray generator equipped with Cu \( K_{\alpha } \) radiation (λ = 0.15406 nm). Diffuse reflectance spectra were measured using a Shimadzu UV-2600 spectrophotometer using the white BaSO4 powder as a reference standard. Photoluminescence emission (PL) and excitation (PLE) were recorded on an F-280 fluorescence spectrophotometer with a 150 W Xe lamp as its excitation source. A homemade temperature control system was used to measure temperature-dependent emission spectra from 298 to 573 K, in which the measuring and controlling accuracy of the temperature is about ± 0.5 °C.
Results and discussion
Phase and crystal structure
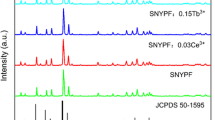
The XRD patterns of Ca9Sr(PO4)6Cl2:5%Ce3+; Ca9Sr(PO4)6Cl2:5%Tb3+; and Ca9Sr(PO4)6Cl2:5%Ce3+, 5%Tb3+ samples are presented in Fig. 1. It can be found that all XRD patterns have the similar profiles, which are well consistent with that of the standard hexagonal Ca10(PO4)6Cl2 reported in ICSD card with the number of 24237 (space group P63/m, no.176). No detectable phase from impurity can be observed in all the as-prepared samples even though Sr2+, Ce3+, and Tb3+ ions were introduced into the host lattice. This suggests that all of the samples are of single phase and the Sr2+, Ce3+, and Tb3+ ions have been successfully incorporated in the Ca10(PO4)6Cl2 host lattice without changing the crystal structure. In addition, we can also see that the XRD peaks of the as-prepared samples are intense and sharp, indicating that the products are crystallized well. This is in the favor of highly efficient luminescence of rare earth ions as well.
Diffuse reflectance spectra
Figure 2 shows the diffuse reflectance spectra of Ca9Sr(PO4)6Cl2, Ca9Sr(PO4)6Cl2:5%Ce3+, Ca9Sr(PO4)6Cl2:5%Tb3+, and Ca9Sr(PO4)6Cl2:5%Ce3+, 5%Tb3+ phosphors, which are good evidence of absorption in the n-UV region induced by the activator ions. For Ca9Sr(PO4)6Cl2 host, it can be found that a very weak absorption band ranges from 230 to 420 nm. When Ce3+ ions are introduced into the host, a strong and broad absorption band in the range of 230–500 nm can be observed, which is ascribed to the 4f–5d absorption of Ce3+ ion. For Ca9Sr(PO4)6Cl2:5%Tb3+, the spectral profile is similar to that of the undoped host except a strong absorption peak at around 250 nm, which corresponds to the 4f-5d transition of Tb3+ ion [12]. However, as Ce3+ and Tb3+ ions are introduced into the host together, the intensity of absorption band of Ce3+ ions are improved obviously, reflecting the occurrence of an energy transfer from Ce3+ to Tb3+. Therefore, the above results also suggest that Ce3+–Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphor can be used as a blue-green tunable phosphor for n-UV LED because of an efficient absorption in the n-UV region.
Photoluminescence properties and concentration quenching of Ce3+ ions
Figure 3a shows the photoluminescent emission (PL) and excitation (PLE) spectra of the Ca9Sr(PO4)6Cl2:5%Ce3+ phosphor. It can be found that the PL spectrum shows a broad nonsymmetric blue emission band extending from 360 to 700 nm with a peak centered at about 431 nm. Since there are two Ca2+ sites in the Ca9Sr(PO4)6Cl2 host and Ce3+ usually presents typical double emission peaks in a single definite lattice site from the lowest 5d excited state to the 2F J (J = 5/2, 7/2) spin–orbit split 4f ground states, the emission spectrum can be decomposed into four Gaussian components with peaks centered at 387, 418, 444, and 487 nm, which are labeled as band A, B, C, and D, respectively [9, 21, 23]. The energy gaps between A and B, and C and D are 1950 and 1989 cm−1, respectively, which is in good agreement with the theoretical value of about 2000 cm−1 [27, 28]. From the emission spectrum, therefore, we concluded that there are two kinds of Ce3+ luminescent centers in the Ca9Sr(PO4)6Cl2 host lattice. Monitoring the emission at 431 nm, the PLE spectrum exhibits a broad band in the range from 220 to 385 nm, which is derived from the 4f–5d transition of Ce3+ ion. The strongest absorption band peaked at about 324 nm, which matches well with n-UV LED chips.
a PL and PLE spectra of Ca9Sr(PO4)6Cl2:5 mol % Ce3+ phosphor and four Gaussian fitting emission spectra. b Normalized PL spectra of Ca9Sr(PO4)6Cl2:xmol%Ce3+ phosphors with different Ce3+ doping concentrations. The inset shows the dependence of luminescent intensity on the doping concentration of Ce3+ ions
In order to investigate the effect of doping concentration on the luminescent properties, a series of Ca9Sr(PO4)6Cl2:x%Ce3+ (\( 1 \le \;x\; \le \;7 \)) phosphors were synthesized. The inset of Fig. 3b shows the dependence of emission intensity on the concentrations of Ce3+ ions in Ca9Sr(PO4)6Cl2 phosphors. It can be observed that the blue emission of the Ce3+ gradually increases with increasing Ce3+ concentration, and reaches a maximum value at x = 5. Continuing the doping concentration of Ce3+ ions, we can find that emission intensity decreases because of the concentration quenching effect. In the meantime, an obvious redshift of the emission peak wavelength is observed in Fig. 3b as the concentration of Ce3+ gradually increases. This phenomenon can be explained as follows. In our case, we propose that there exists an energy transfer between Ce3+ ions in different crystallographic sites. Thus, the intensities of four Gaussian components (CeA–CeD) originated from two kinds of Ce3+ increased relatively with increasing Ce3+ concentration, and it affected the change of emission shape and the peak position by the substitution of Ca2+ ions [6, 11, 28]. Furthermore, the reabsorption of the high-energy part of the emission (resonant with the low-energy part of the excitation spectra) is another possible reason for the redshift of the emission spectrum in Ca9Sr(PO4)6Cl2:x%Ce3+ (\( 1 \le \;x\; \le \;7 \)) phosphors [29].
Usually, it is considered that concentration quenching is mainly caused by energy transfer among Ce3+ ions. Therefore, to further investigate the energy transfer mechanism of Ce3+ ions in Ca9Sr(PO4)6Cl2, the critical distance of energy transfer (R c) should be evaluated first. According to the theory proposed by Blasse, the critical distance, R c, can be expressed as follows [29, 30]:
where V is the volume of the unit cell, x C is the critical concentration of Ce3+ ions, and N is the number of host cations in the unit cell. As for Ca9Sr(PO4)6Cl2 host, V = 537.64 Å3, x C = 0.05, and N = 10. Therefore, the critical transfer distance R c is calculated to be 12.71 Å. Non-radiative energy transfer may occur by exchange interaction or multi-polar interaction. The former one is dominant when the critical distance is <4 Å [30, 31]. So it can be excluded that the exchange interaction is responsible for the energy transfer between Ce3+ ions in Ca9Sr(PO4)6Cl2 host. Therefore, electric multipole should be the dominant mechanism for the energy transfer between Ce3+ ions in Ca9Sr(PO4)6Cl2:Ce3+ phosphors. Van Uitert has developed a phenomenological model to explain the relationship between the luminescent intensity and the concentration of luminescent center, which can be written as [32, 33]
where x is the doping concentration of Ce3+ ions in the present case; K and β are constants for a certain system; θ represents the interaction type between luminescence center and quenching center, here θ = 6, 8, or 10, indicating the exchange interaction, electric dipole–dipole (D–D), electric dipole–quadrupole (D–Q), and electric quadrupole–quadrupole (Q–Q) interactions, respectively. In order to understand the energy transfer mechanism between Ce3+ ions in Ca9Sr(PO4)6Cl2 phosphors, Eq. (2) was used to fit the experimental data in Fig. 4. It can be found that Eq. (2) fit well with the experimental data, and the θ value was deduced from the fitting process to be 6.21, which suggests that the electric dipole–dipole interaction should be responsible for the energy transfer of Ce3+ in Ca9Sr(PO4)6Cl2 phosphors.
Energy transfer from Ce3+ to Tb3+
As for the Tb3+-doped Ca9Sr(PO4)6Cl2 sample, its PLE and PL spectra are presented in Fig. 5b. The PL spectrum under the excitation of 379 nm displays a series of sharp-line emissions. The emission peaks at 486, 541, 590, and 618 nm are assigned to the 5D4–7F J (J = 6, 5, 4, 3) characteristic transitions of Tb3+ ion. The green emission line at 541 nm from the 5D4–7F5 transitions dominates the whole emission spectrum, which is a magnetic dipole transition with \( \Delta J\; = \;1 \) [9, 34]. Therefore, the Tb3+-doped Ca9Sr(PO4)6Cl2 sample exhibits green emission under the excitation of 379 nm. Monitoring the emission at 541 nm, the PLE spectrum contains a broad band and several lines. The broad band ranging from 200 to 270 nm centered at 256 nm is assigned to the 4f–5d transition of Tb3+ ions, while the excitation lines are attributed to the intra-4f 8 transitions of Tb3+ ions [2]. Comparing the PL spectra of Ce3+ (Fig. 5a) with the PLE spectra of Tb3+ (Fig. 5b), it is clearly observed that there is a spectral overlap, indicating that an effective energy transfer from the sensitizer, Ce3+, to the activator, Tb3+, can be expected in the Ca9Sr(PO4)6Cl2 host. Meanwhile, as shown in Fig. 5c, it can be found that the profile of PLE spectrum of Ce3+–Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphor monitored the emission of Tb3+ ions at 541 nm is similar to that monitored the emission of Ce3+ ions at 431 nm. Moreover, when the sample was excited by 324 nm UV light, the PL spectrum shows the weaker blue emission of Ce3+ and the stronger green emission of Tb3+ comparing with that in Ca9Sr(PO4)6Cl2:Ce3+ and Ca9Sr(PO4)6Cl2:Tb3+, which is an obvious evidence for the energy transfer from Ce3+ to Tb3+ in Ca9Sr(PO4)6Cl2 host [35]. The PLE spectra consist of a broad band extending from 250 to 360 nm which means that Ca9Sr(PO )4 6Cl2:Ce3+,Tb3+ phosphor is a potential green-emitting phosphor for UV pumped WLEDs as well.
Figure 6 shows the PL spectra of the as-prepared Ca9Sr(PO4)6Cl2:5%molCe3+, y%molTb3+ (0 ≤ y ≤ 7) samples under the excitation of 324 nm. It can be seen that all the emission spectra mainly consist of a broad emission band of several line peaks. This form corresponds to the 4f–5d transitions of Ce3+ ion and the later originates from the 5D4–7F J (J = 6, 5, 4, 3) characteristic transitions of Tb3+ ion. Moreover, the emission intensity of the Ce3+ decreases monotonously with increasing the Tb3+ concentration when the Ce3+ doping concentration is fixed, which indicates an efficient energy transfer from Ce3+ to Tb3+. From the luminescence of Tb3+ ions, it can be found that the emission intensity of Tb3+ increases gradually and reaches a maximum value when y = 5. As a result of concentration quenching, the emissions of Tb3+ decreases when the Tb3+ ion concentration exceeds over 5 %.
In general, the energy transfer efficiency from a sensitizer to activator, η ET, can be expressed as the following equation [36]:
where I s is the luminescent intensity of Ce3+ in the presence of Tb3+ and I s0 is the luminescent intensity of Ce3+ in the absence of Tb3+. In the Ca9Sr(PO4)6Cl2:Ce3+, Tb3+ system, Ce3+ is the sensitizer and Tb3+ is the activator. Figure 7 shows the result of energy transfer efficiency from Ce3+ to Tb3+ calculated using Eq. (3). As shown in Fig. 7, the energy transfer efficiency increases with the increase of Tb3+ concentration. However, the increscent rate of the emission intensity gradually decreases with increasing Tb3+ concentration. The maximal energy transfer efficiency can reach 76 % when 7 %mol Tb3+ ions were doped. The above results indicate that the energy transfer from Ce3+ to Tb3+ is very efficient.
On the basis of Dexter’s energy transfer formula of multi-polar interaction and Reisfeld’s approximation, the following relation can be obtained [34–37]:
where \( \eta_{0} \) and \( \eta \) are the luminescent quantum efficiency of the sensitizer (Ce3+) in the absence and presence of an activator (Tb3+), respectively and C is the concentration of Tb3+ ions. The value of \( \eta_{0} /\eta \) was estimated by the ratio of luminescence intensities as [36–38]
where I 0 and I S are the intrinsic luminescence intensity of a sensitizer (Ce3+) in the absence and presence of an activator (Tb3+), respectively; n = 6, 8, and 10 corresponding to dipole–dipole (d–d), dipole–quadrupole (d–q), and quadrupole–quadrupole (q–q) interactions, respectively. The I S0 /I S ~ C n/3 plots are illustrated in Fig. 8. Best linear behavior can be observed only when n = 8, indicating that energy transfer from Ce3+ to Tb3+ occurred via the electric dipole–quadrupole interaction (d–q) mechanism in the Ca9Sr(PO4)6Cl2 host.
Color chromaticity
Because the spectral components can be greatly changed with the increase of Tb3+ concentration, as a result, the PL emitting colors can be tuned. In order to more intuitionistically observe the effect of Ce3+ doping concentration on PL emission colors, the Commission International del’Eclairage (CIE) chromaticity coordinates of Ca9Sr(PO4)6Cl2:5%Ce3+, y%Tb3+ (0 ≤ y ≤ 7) phosphors were calculated and the typical results are shown in Table 1 and Fig. 9. It can be seen that the chromaticity coordinates tune from (0.169, 0.151) to (0.258, 0.460) with increasing the concentration of Tb3+ from 0 to 7 %. Correspondingly, the color tone of the phosphors can be adjusted from indigo to green under the excitation of UV light, which suggests that the phosphor may have potential application for n-UV pumped WLEDs.
Thermal quenching
For the application of high-power WLEDs, thermal stability is one of the most important parameters for phosphors, because it can considerably influence the light output, color rendering index, and stability of devices. Herein, temperature-dependent emission spectra of the selected Ca9Sr(PO4)6Cl2:5%Ce3+, 5%Tb3+ phosphor at different temperatures from 298 to 573 K under the excitation of 324 nm are shown in Fig. 10a. It is obvious that the emission intensities of the sample decrease with increasing temperature from 298 to 573 K. Especially, the PL intensity at 150 °C drops to be around 57.6 % of the initial value at room temperature, which suggests that the as-prepared phosphors possesses good thermal stability properties (as shown in the inset of Fig. 10a). Generally, the decrease of emission intensity is ascribed to the thermal quenching of emission intensity via phonon interaction, in which the excited luminescent center is thermally activated through the crossing point between the ground and the excited states [39, 40]. In order to calculate the activation energy (ΔE) for thermal quenching and to better understand the thermal quenching process, the temperature-dependent emission intensity is described by a modified Arrhenius equation [6, 41, 42]:
aThe PL spectra (\( \lambda_{\text{ex}} = 324{\text{ nm}} \)) of Ca9Sr(PO4)6Cl2:5mol%Ce3+, 5mol%Tb3+ phosphor at different temperatures from 298 K to 573 K. The inset shows the relative intensity as a function of temperature of the selected phosphor. b A fitting line of ln[(I 0 /I)−1] vs.1/kT activation energy graph for thermal quenching of the corresponding sample
Herein, I(T) and I 0 are the intensities of the initial and different temperatures, respectively; c is a constant; k is the Boltzmann’s constant (8.617 × 10−5 eV); and ΔE is the activation energy for thermal quenching. So Eq. (6) can be rewritten as
According to Eq. (7), the activation energy ΔE can be calculated from a plot of ln[(I 0 /I)−1] against 1/kT. As shown in Fig. 10b, a linear fitting can be obtained using Eq. (7). The slope of the linear line equals −ΔE. Thus, the ΔE was deduced to be 0.176 eV.
Conclusions
To sum up, a series of Ce3+-doped and Ce3+/Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphors have been prepared via solid-state reaction successfully. The optimal doping concentration of Ce3+ ions in Ca9Sr(PO4)6Cl2 phosphor was confirmed to be 5 mol%. Moreover, an obvious redshift of emission band was observed with the increase of Ce3+. For the Ce3+/Tb3+ co-doped Ca9Sr(PO4)6Cl2 phosphor, the emission intensity of Ce3+ ions dramatically decreases with the increase of Tb3+ ion concentration because of an energy transfer from Ce3+ to Tb3+. Moreover, the energy transfer efficiency increases greatly with the increase of Tb3+ ion concentration. The mechanism of energy transfer between Ce3+ and Tb3+ was deduced to be electric dipole–dipole–quadrupole interaction. The results of temperature-dependent emission spectra indicate that the Ca9Sr(PO4)6Cl2: Ce3+,Tb3+ phosphor has excellent thermal stability of luminescence. The results above suggest that the as-prepared phosphors can be used as blue and green components for n-UV pumped WLEDs.
References
Radkov E, Bompiedi R, Srivastava AM, Setlur AA, Becker C (2004) White light with UV LEDs. Proc SPIE 5187:171–177
Liang C, You HP, Fu YB, He JH (2015) A novel tunable blue-green-emitting CaGdGaAl2O7:Ce3+, Tb3+ phosphor via energy transfer for UV-excited white LEDs. Dalton Trans 44:8100–8106
Im WB, Brinkley S, Hu J, Mikhailovsky A, DenBaars SP, Seshadri R (2010) Sr2.97−xBaxCe0.025AlO4F: a highly efficient green-emitting oxyfluoride phosphor for solid state white lighting. Chem Mater 22:2842–2849
Guo CF, Jing H, Li T (2012) Green-emitting phosphor Na2Gd2B2O7:Ce3+, Tb3+ for near-UV LEDs. RSC Adv 2:2119–2122
Wu JL, Gundiah G, Cheetham AK (2007) Structure-property correlations in Ce-doped garnet phosphors for use in solid state lighting. J Chem Phys Lett 441:250–254
Lee GY, Han JY, Im WB, Cheong SH, Jeon DY (2012) Novel blue-emitting Na x Ca1−x Al2−x Si2+x O8:Eu2+ (x = 0.34) phosphor with high luminescent efficiency for UV-pumped light-emitting diodes. Inorg Chem 51:10688–10694
Lin HC, Yang CY, Das S, Chung-Hsin Lu (2014) Photoluminescence properties of color-tunable Ca3La6(SiO4)6:Ce3+, Tb3+ phosphors. J Am Ceram Soc 97:1866–1872
Duan CJ, Zhang ZJ, Rosler S, Delsing A, Zhao JT, Hintzen HT (2011) Preparation, characterization, and photoluminescence properties of Tb3+, Ce3+, and Ce3+/Tb3+-activated RE2Si4N6C (RE = Lu, Y, and Gd) phosphors. Chem Mater 23:1851–1861
Cao CY, Yang HK, Chung JW, Moon BK, Choi BC, Jeong JH, Kim KH (2011) Hydrothermal synthesis and enhanced photoluminescence of Tb3+ in Ce3+/Tb3+ doped KGdF4 nanocrystals. J Mater Chem 21:10342–10347
Maggay IVB, Lin PC, Liu WR (2015) Investigation of luminescence properties and the energy transfer mechanism of Li6Lu(BO3)3:Ce3+, Tb3+ green-emitting phosphors. RSC Adv 5:5591–5597
Xia ZG, Wu WW (2013) Preparation and luminescence properties of Ce3+ and Ce3+/Tb3+-activated Y4Si2O7N2 phosphors. Dalton Trans 42:12989–12997
Nohara A, Takeshita S, Isobe T (2014) Mixed-solvent strategy for solvothermal synthesis of well-dispersed YBO3:Ce3+, Tb3+ nanocrystals. RSC Adv 4:11219–11224
Chen MY, Xia ZG, Liu QL (2015) Luminescence properties and energy transfer of Ce3+/Tb3+ co-doped Ca6Ba(PO4)4O phosphor for near-UV pumped light-emitting diodes. J Mater Chem C 3:4197–4204
Park WB, Singh SP, Pyo M, Sohn KS (2011) Y6+x/3Si11−yAlyN20+x−yO1−x+y:Re3+ (Re = Ce3+, Tb3+, Sm3+) phosphors identified by solid-state combinatorial chemistry. J Mater Chem 21:5780–5785
Sayed FN, Grover V, Godboleb SV, Tyagi AK (2012) Color tunable YF3:Ce3+/Ln3+ (Ln3+: Eu3+, Tb3+, Dy3+, Sm3+) luminescent system: role of sensitizer and energy transfer study. RSC Adv 2:1161–1167
Jia D, Meltzer RS, Yen WM, Jia W, Wang X (2002) Green phosphorescence of CaAl2O4:Tb3+, Ce3+ though persistence energy transfer. Appl Phys Lett 80:1535–1537
Zhou XF, Zhang ZY, Wang YH (2015) Ce3+ and Tb3+ singly- and co-doped MgGd4Si3O13 for ultraviolet light emitting diodes and field emission displays. J Mater Chem C 3:3676–3683
Shmulovich J, Berkstresser GW, Brasen D (1985) Tb3+-Ce3+ energy transfer in Tb3+/Ce3+: YAG single crystals. J Chem Phys 82:3078–3082
Blesse G, Bril A (1967) Study of energy transfer from Sb3+, Bi3+, Ce3+ to Sm3+, Eu3+, Tb3+, Dy3+. J Chem Phys 47:1920–1926
Sahu NK, Singh NS, Pradhan L, Bahadur D (2014) Ce3+ sensitized GdPO4:Tb3+ with iron oxide nanoparticles: a potential biphasic system for cancer theranostics. Dalton Trans 43:11728–11738
Pazik R, Nedelec JM, Wiglusz RJ (2014) Preferential site substitution of Eu3+ ions in Ca10(PO4)6Cl2 nanoparticles obtained using a microwave stimulated wet chemistry technique. CrystEngComm 16:5308–5318
Notzold D, Wulff H, Herzo G (1995) Structural and optical properties of the system (Ca, Sr, Eu)(PO4)3Cl. Phys Stat Sol 191:21–30
Wang CH, Gui DY, Qin R, Yang FL, Jing XP, Tian GS, Zhu WJ (2013) Site and local structure of activator Eu2+ in phosphor Ca10−x(PO4)6Cl2:xEu2+. J Solid State Chem 206:69–74
Babu R, Jena H, Govindan Kutty KV, Nagarajan K (2011) Thermodynamic functions of Ba10(PO4)6Cl2, Sr10(PO4)6Cl2 and Ca10(PO4)6Cl2. Thermochim Acta 526:78–82
Fu ZL, Wang XJ, Yang Y, Wu ZJ, Duana DF, Fu XH (2014) Hydrothermal synthesis, electronic structure and tunable luminescence of single-phase Ca5(PO4)3F:Tb3+/Eu3+ microrods. Dalton Trans 43:2819–2827
Zhou L, Liang HB, Peter AT, Zhang Su, Hou DJ, Liu CM, Tao Y, Huang Y, Li LN (2013) Luminescence, cathodoluminescence and Ce3+/Eu2+ energy transfer and emission enhancement in the Sr5(PO4)3Cl:Ce3+, Eu2+ phosphor. J Mater Chem C 1:7155–7165
Wu JL, Gundiah G, Cheetham AK (2007) Structure-property correlations in Ce-doped garnet phosphors for use in solid state lighting. Chem Phys Lett 441:250–254
Zhang XG, Gong ML (2014) Single-phased white-light-emitting NaCaBO3:Ce3+, Tb3+, Mn2+ phosphors for LED applications. Dalton Trans 43:2465–2472
Wang DY, Huang CH, Wu YC, Chen TM (2011) BaZrSi3O9:Eu2+: a cyan-emitting phosphor with high quantum efficiency for white light-emitting diodes. J Mater Chem 21:10818–10822
Blasse G (1969) Energy transfer in oxidic phosphors. Philips Res Rep 24:131–136
Antipeuko BM, Bataev IM, Ermolaev VL, Privalova TA (1970) Ion-to-ion radiationless transfer of electron excitation energy between rare-earth ions in POCl3-SnCl4. Opt Spektrosk 29:335
Ozawa L, Jaffe PM (1971) The mechanism of the emission color shift with activator concentration in Eu3+ activated phosphors. J Electrochem Soc 118:1678–1679
Van Uitert L (1976) Characterization of energy transfer interactions between rare earth ions. J Electrochem Soc 114:1048–1053
Cheng SD, Kam CH, Buddhudu S (2001) Enhancement of green emission from Tb3+: GdOBr phosphors with Ce3+ ion co-doping. Mater Res Bull 36:1131–1137
Dong J, Wang L, Cui CE, Tian Y, Huang P (2015) Luminescence properties of Ce3+-doped and Ce3+-Tb3+ co-doped Na0.34Ca0.66Al1.66Si2.34O8 phosphor for UV-LED. Ceram Int 41:1341–1346
Bourcet JC, Fong FK (1974) Quantum efficiency of diffusion limited energy transfer in La1−x−yCexTbyPO4. J Chem Phys 60:34–39
Dexter DL, Schulman JH (1954) Theory of concentration quenching in inorganic phosphors. J Chem Phys 22:1063–1070
Reisfeld R, Greenberg E, Velapoldi R, Barnett B (1972) Luminescence quantum efficiency of Gd and Tb in borate glasses and the mechanism of energy transfer between them. J Chem Phys 56:1698–1705
Hsu CH, Lu CH (2011) Structural and optical characteristics of CeSi3N5:Tb3+ nitridosilicate phosphors. J Am Ceram Soc 94:1320–1323
Reisfeld R, Lieblich-Soffer N (1979) Energy transfer from UO2 2+ to Sm3+ in phosphate glass. J Solid State Chem 28:391–395
Blasse G, Grabmaier BC (1994) Luminescent Materials. Springer, Berlin, pp 91–107
Geng K, Xia ZG, Molokeev MS (2014) Crystal structure and luminescence property of a novel blue-emitting Cs2xCa2xGd2(1−x)(PO4)2:Eu2+ (x = 0.36) phosphor. Dalton Trans 43:14092–14098
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (NSFC, 51302182), the National High Technology Research and Development Program (“863” Program) of China (2015AA016901), the Qualified Personnel Foundation of Taiyuan University of Technology (QPFT) (No: tyut-rc201361a), and the Program for the outstanding Innovative Teams of Higher Learning Institutions of Shanxi.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Feng, L., Tian, Y., Wang, L. et al. Tunable emission, energy transfer, and thermal stability of Ce3+-doped and Ce3+/Tb3+ Co-doped Ca9Sr(PO4)6Cl2 phosphors. J Mater Sci 51, 2841–2849 (2016). https://doi.org/10.1007/s10853-015-9592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9592-x