Abstract
Nanocrystalline CuInS2 particles have been synthesized from copper, indium, and sulfur powders by high-energy milling in a planetary mill in an argon atmosphere. Structural characterization of the prepared nanoparticles, including phase identification, Raman spectroscopy, specific surface area measurement, and particle size analysis were performed. The optical properties were studied using UV–Vis absorption and photoluminescence (PL) spectroscopy. The production of CuInS2 (JCPDS 027-0159) particles with a crystallite size of about 17.5–23.5 nm was confirmed by X-ray diffraction. The crystal structure has a tetragonal body-centered symmetry belonging to the I-42d space group. The Raman spectra also proved the formation of pure CuInS2 nanoparticles. TEM and HRTEM measurements revealed the presence of nanoparticles of different dimensions (10–20 nm) and their tendency to form agglomerates. The nanoparticles tend to agglomerate due to their large specific surface area. The average size of the synthesized particles was determined by photon cross-correlation spectroscopy to be in the range of 330–530 nm (bimodal size distribution). The band gap of the CuInS2 particles is 2 eV which is wider than that in bulk materials. The decrease in size leads to the blue-shift of the PL spectra. Therefore, CuInS2 nanoparticles are promising candidates for optical applications, and they have high potential in solar energy conversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ternary semiconductors of the I–III–VI2 group have received considerable attention over the last decades due to their potential technological applications such as photoelectrochemical cells, photoconductivity characteristics, and other beneficial intrinsic properties. They are actively used in the optoelectronic and solar cell applications [1–4]. However, many of the best studied nanocrystalline materials contain toxic heavy metals; this seriously limits their potential for widespread application. Copper indium disulfide CuInS2 is a selenium-free compound from the chalcopyrite family, and its development is attractive because the problematic selenium is substituted by non-toxic sulfur in this case. Bulk CuInS2 is a semiconductor with a direct optical band gap of 1.5 eV (820 nm) and with high absorption coefficient of the order of 10−5cm−1 [5].
Among different materials, CuInS2 may be a promising semiconductor material due to its applications in the area of visible and infrared light-emitting diodes, solar cells, infrared detectors, optical parametric oscillators, upconverters, far-infrared generators, etc. [6–9]. There are various techniques [10–18] for the synthesis of CuInS2 powders and thin films with different nano- and microforms, such as nanoparticles [12], nanorods [14], porous microspheres [15], nanotubes [16], foam-like nanocrystallites [17], and nanowires [18], including chemical vapor deposition (CVD), solvothermal route, spray pyrolysis, vacuum evaporation, sputtering, and thermal evaporation.
Mechanochemistry offers new challenges in the synthesis of nanocrystals [19, 20]. This synthetic route can be performed via simple solid-state approach using ambient pressure and room temperature.
This paper is focused on the synthesis and study of structural, surface, and optical properties of mechanochemically synthesized CuInS2 nanoparticles from the point of view of the possible application. The controlled mechanochemical synthesis of nanocrystalline CuInS2 at ambient temperature in the absence of a solvent is still a great challenge.
Experimental
Mechanochemical synthesis of CuInS2 was performed in a planetary ball mill (Pulverisette 6, Fritsch, Germany) starting from powders of copper (99.7 %, Aldrich, Germany), indium (99.99 %, Aldrich, Germany), and sulfur (99 %, Ites, Slovakia) in an argon atmosphere, according to the reaction:
The milling was performed in a 250-ml tungsten carbide milling chamber with 50 tungsten carbide balls, 10 mm in diameter. The rotational speed of the planet carrier was 550 rpm. The powder charge was 1.31 g Cu, 2.37 g In, and 1.32 g S, corresponding to the stoichiometry of Eq. (1). The milling time was 30–120 min and the mill operated at room temperature.
The X-ray diffraction patterns were obtained using a D8 Advance diffractometer (Bruker, Germany) in the Bragg–Brentano geometry, working with a CuKα radiation and a scintillation detector. For the data treatment, the commercial Bruker processing tools have been used. Specifically, for phase identification the Diffracplus Eva and for Rietveld analysis the DiffracplusTopas software have been utilized.
The micro-Raman measurements were performed in air at room temperature, with the focus of the beam of an Ar laser (514 nm) via a confocal Raman Microscope (Spectroscopy & Imaging, Germany) in backscattering geometry. The frequency of the Raman line of crystalline Si at 520 cm−1 was used to calibrate the system in the present study.
The specific surface area was determined by the low-temperature nitrogen adsorption method in a Gemini 2360 sorption apparatus (Micromeritics, USA).
The particle size distribution was measured by photon cross-correlation spectroscopy (PCCS) using a Nanophox particle size analyzer (Sympatec, Germany). A part of the sample was added into distilled water and sonicated for 10 min. Then the sample was further diluted, in order to achieve a suitable concentration for the measurement by PCCS. The analyses were performed with a dispersant refractive index of 1.33 and in the range 10–10,000 nm. The measurements were performed 3 times for each sample.
The morphology and microcharacterization of the sample were analyzed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) techniques. A small quantity of the powder sample was deposited on carbon-coated copper grids (for SEM) or nickel grids (for TEM, to avoid the interference between the Cu grid and the Cu from the sample in the EDS analysis). A MIRA3 FE-SEM microscope (TESCAN, Czech Republic) equipped with EDX detector (Oxford Instrument, United Kingdom) was used. The sample was sufficiently conductive, thus it is not coated with any conductive material in order to avoid charging artifacts. An FEI TECNAI G2 F30 S-twin high-resolution transmission electron microscope (HRTEM) was used, with field emission gun (FEG) and scanning transmission capabilities (STEM) operating at 300 kV and 0.2 nm point resolution, equipped with a high-angle annular dark-field (HAADF) detector with 0.16 nm point resolution from Fischione Instruments, and an INCA X-Max 80 silicon drift detector (SDD) for the energy-dispersive X-ray analysis (EDX). The Gatan Digital Micrograph software (Gatan Inc.) was used to measure the lattice spacing and to calculate the First Fourier Transform (FFT). The analysis of the HAADF-STEM images and the EDX profile was done with the ES Vision software (FEI Company).
The absorption spectra were measured using an UV–Vis spectrophotometer Helios Gamma (Thermo Electron Corporation, Great Britain) in quartz cell by dispersion of the synthesized particles in absolute ethanol by ultrasonic stirring.
The micro-PL spectra were measured by an UV–Vis–NIR confocal Raman Microscope (Spectroscopy & Imaging, Germany). The excitation at 514 nm was carried out using an Ar laser. For measuring the PL intensity, the nanopowders were dispersed on SiO2/Si substrate.
Results and discussion
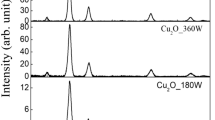
The progress of the mechanochemical synthesis of CuInS2 is illustrated by XRD patterns of samples taken after 30–120 min of milling (a–c) (Fig. 1). The mechanochemical reaction is completed after 30 min of milling. The product was identified via XRD analysis as roquesite (JCPDS 027-0159). The crystal structure has a tetragonal body-centered symmetry belonging to the I-42d space group with the lattice parameters a = b = 5.523 Å and c = 11.141 Å. The characteristic peaks of other impurities were not detected. The most intense peak appeared at 27.9° is attributed to the chalcopyrite phase oriented along the [112] crystal plane. The estimated average crystallite size is 23.5 nm for the CuInS2 milled for 30 min. Milling for 60 min leads to obvious but not too dramatic structural refinement, and the crystallite size decreases to 18 nm. No considerable changes can be observed after milling for 120 min (crystallite size is 17.5 nm).
Raman spectroscopy confirmed the pure crystalline nanoparticle formation. In Fig. 2, the micro-Raman spectra of the synthesized sample are given. The figure shows that the dominant features of Raman plots are intense peaks at 298 cm−1 and the weaker peaks at 240 and 340 cm−1, which were previously assigned to the A1, E, and B2 modes of CuInS2 phase, respectively [9, 21]. No other Raman peaks were observed, indicating pure CuInS2 nanoparticle growth. It can be also seen that at 40 % excitation power recrystallization of the structure occurs, manifested by increase and narrowing of the peak at 298 cm−1 and decrease of the other peaks in comparison with the Raman spectra measured at only 20 % excitation power by laser light.
The high-energy milling of solids is accompanied by an increase in the number of nanoparticles and by generation of fresh, previously unexposed surface. Specific surface area values belong to one of the most important characteristics of the milled samples [19]. The specific surface area of the mechanochemically synthesized CuInS2 nanoparticles was in the range 3.4–4.0 m2 g−1. Particles in the nanometer size range have a strong tendency to agglomerate due to their relatively large specific surface area.
The particle size distribution of the nanocrystalline CuInS2 nanoparticles was characterized using a photon cross-correlation spectroscopy as shown in Fig. 3. The size dispersion has a relatively narrow, bimodal distribution profile, with an average particle size in the range of 250–670 nm, indicating that relatively large agglomerates of CuInS2 resulted from high-energy ball milling.
The surface morphology of the mechanochemically synthesized CuInS2 was pursued using SEM. The micrographs are presented in Fig. 4.
As can be seen from the microscopic point of view (left part of Fig. 4a), the tendency toward agglomeration is evident. After 60 min of milling, together with the presence of larger grains, also very fine grains occur. Very fine particles can be observed, and although agglomerates are formed, these fine particles belonging to nano-region are retained. To confirm the presence of nanoparticles definitely, even higher magnification was used (Fig. 4b). It can be concluded that the CuInS2 sample exhibits agglomerated grains with large amount of nanoparticles. From SEM images, it is also evident that particles are clusters of tiny crystallites.
The EDS layered image comprising all the elements is illustrated in Fig. 5a. The energy-dispersive X-ray spectroscopy (EDS) elemental mapping for the mechanochemically synthesized CuInS2 is shown in Fig. 5b–d. It can be clearly seen that all constituent elements are uniformly distributed over the as-prepared sample confirming the homogeneity of the powder. In the EDX spectra (Fig. 5e), the same elements can be observed. The results confirm the presence of the elements from which the mechanochemically synthesized CuInS2 is formed and a significant amount of copper (26.5 at.%), indium (24.9 at.%.), and sulfur (48.6 at.%) is evidenced. However, it has to be noted that synthesized product is of the desired purity.
The microstructure of the CuInS2 sample after 60 min of milling was further studied using TEM, HRTEM, EDS, and HAADF-STEM techniques, and the obtained results are depicted in Fig. 6. In the TEM image (Fig. 7a), it was found that the sample is formed by quite large particles (100–300 nm), and this result is in good agreement with that determined by photon cross-correlation spectroscopy. However, the HRTEM images show that those particles are formed by agglomerated nanocrystals of CuInS2 with very small crystallite size (10–20 nm), confirming the results of XRD analysis.
Microcharacterization of CuInS2 sample synthesized mechanochemically during 60 min. a TEM image; b–d HRTEM micrographs; e HAADF-STEM image, the line profile analysis is marked with a red line; f EDX spectrum taken in the first dot of the scanning image. g Representation of the Cu–In–S EDS signal in each point of the line profile
In some particle edges, the initial crystallization and some amorphous areas can be observed (Fig. 6b). Some oriented nanocrystals were elucidated from the contrast (marked with a white square) in the images presented in Fig. 6c, d, and the first Fourier transform (FFT) was done to estimate the zone axis of the orientation. The results show the [110], [201], and [531] zone axes of the tetragonal roquesite system. The Cu, In, and S atomic percentages were analyzed by EDX technique in the STEM mode with an HAADF detector. A line profile analysis was done through the red line marked in the HAADF-STEM image (Fig. 6e) and five dots were analyzed. The EDX spectrum taken in the first point is depicted in Fig. 6f and the S–K, Cu–K, L, and In–L edges are well observed. A resume of the S–Cu–In signal through the line profile is represented in Fig. 6g.
As the size of the nanoparticles decreases, the band gap increases and the absorption and photoluminescence spectra shift to shorter wavelengths. Figure 7 shows the optical absorption properties of the mechanochemically synthesized (60 min) CuInS2 investigated by UV–Vis spectroscopy. The band gap of the CuInS2 is approximated using the direct band method, by plotting the squared absorbance versus energy and extrapolating to zero, as shown in the inset. The band gap of CuInS2 is estimated to be 2.0 eV, which is in good agreement with the previous reports [12, 22, 23]. The observed peak is blue-shifted relative to the bulk CuInS2 at 1.5 eV [5] and is assigned to the optical transitions of the excitonic states in CuInS2. The obvious blue-shift could be attributed to the existence of very small CuInS2 nanocrystalline particles agglomerated into the large clusters.
The micro-PL spectrum of the mechanochemically synthesized nanocrystallites is shown in Fig. 8. With the excitation wavelength at 514 nm, the corresponding emission peaks were found at around 700 nm (1.77 eV) and 900 nm (1.37 eV). The peak observed at 700 nm is in accordance with the literature [24]. The broad PL emissions are typically observed in chalcopyrite I–III–VI2-based semiconductor nanocrystals. The radiative recombination of excited electron–hole pairs in such nanocrystals is associated with deep defect states inside the band gap, being referred to as donor–acceptor pair recombination [5]. Probably, the peak at 900 nm (1.37 eV) may be attributed to a transition from shallow trap centers to the conduction band [25].
As can be seen (Figs. 7, 8), the decreasing size of CuInS2 leads to the blue-shift of the absorption and PL spectra. In addition, the PL spectrum is red-shifted compared to the absorption spectrum, which indicates the large Stokes shift of the nanoparticles.
Conclusion
Nanocrystalline copper indium disulfide CuInS2 has been synthesized by high-energy ball milling in a planetary mill in an argon atmosphere. The obtained material had a tetragonal crystalline structure. TEM measurements also revealed the presence of nanocrystals with the size of 10–20 nm with the tendency to form agglomerates. The bimodal size distribution profile was found with a mean particle size in the range of 330–530 nm. Using the SEM microscopy, elemental mapping proved the homogeneous distribution of Cu, In, and S elements. Both UV–Vis absorption and PL emission spectra recorded for CuInS2 indicate quantum size effects. Such prepared nanocrystalline CuInS2 has a wider band gap (2 eV) in comparison to the bulk one (1.5 eV) what could be in many aspects of application more beneficial. Mechanochemical synthesis of nanocrystalline ternary metal chalcogenides may be a contribution to nanotechnology and materials engineering. The controlled mechanochemical synthesis can be considered environmentally friendly, because a solvent-free mechanochemical procedure was performed under ambient temperature and atmospheric pressure.
References
Green MA, Emery K, King DL, Igari S, Warta W (2002) Solar cell efficiency tables (version 20). Prog Photovolt 10(5):355–360. doi:10.1002/Pip.453
Pinjari DV, Pandit AB (2010) Cavitation milling of natural cellulose to nanofibrils. Ultrason Sonochem 17(5):845–852. doi:10.1016/j.ultsonch.2010.03.005
Klenk R, Klaer J, Scheer R, Lux-Steiner MC, Luck I, Meyer N, Ruhle U (2005) Solar cells based on CuInS2—an overview. Thin Solid Films 480:509–514. doi:10.1016/j.tsf.2004.11.042
Kazmerski L, Ramanathan K (2005) The 14th international conference on ternary and multinary compounds. J Phys Chem Solids 66(11):1853. doi:10.1016/j.jpcs.2005.10.171
Xiao JP, Xie Y, Tang R, Qian YT (2001) Synthesis and characterization of ternary CuInS2 nanorods via a hydrothermal route. J Solid State Chem 161(2):179–183. doi:10.1006/jssc.2001.9247
Zhou JC, Li SW, Gong XL, Yang YL, Guo Y (2011) Preparation of CuInS2 microspheres via a facile solution-chemical method. Mater Lett 65(12):2001–2003. doi:10.1016/j.matlet.2011.03.089
Kim KH, Lee JK, Alphonse A, Erkan ME, Shin DC, Lim DG, Park BO, Jin MHC (2013) Preparation of precursor particles by cryogenic mechanical milling for the deposition of CuInS2 thin films. Mater Sci Semicond Process 16(1):226–230. doi:10.1016/j.mssp.2012.08.012
Amiri O, Salavati-Niasari M, Sabet M, Ghanbari D (2013) Synthesis and characterization of CuInS2 microsphere under controlled reaction conditions and its application in low-cost solar cells. Mater Sci Semicond Process 16(6):1485–1494. doi:10.1016/j.mssp.2013.04.026
Li DS, Zou Y, Yang DR (2012) Controlled synthesis of luminescent CuInS2 nanocrystals and their optical properties. J Lumin 132(2):313–317. doi:10.1016/j.jlumin.2011.08.030
Scheer R, Klenk R, Klaer J, Luck I (2004) CuInS2 based thin film photovoltaics. Sol Energy 77(6):777–784. doi:10.1016/j.solener.2004.08.004
Long F, Wang WM, Tao HC, Jia TK, Li XM, Zou ZG, Fu ZY (2010) Solvothermal synthesis, nanocrystal print and photoelectrochemical properties of CuInS2 thin film. Mater Lett 64(2):195–198. doi:10.1016/j.matlet.2009.10.044
Han SK, Kong MG, Guo Y, Wang MT (2009) Synthesis of copper indium sulfide nanoparticles by solvothermal method. Mater Lett 63(13–14):1192–1194. doi:10.1016/j.matlet.2009.02.032
Lee DY, Kim J (2010) Characterization of sprayed CuInS2 films by XRD and Raman spectroscopy measurements. Thin Solid Films 518(22):6537–6541. doi:10.1016/j.tsf.2010.03.062
Zhang JJ, Li Q, Chen J (2014) Synthesis and characterization of chalcopyrite CuInS2 nanorods by an organic molten salt method. Mater Lett 120:182–184. doi:10.1016/j.matlet.2014.01.079
Hu HM, Yang BJ, Liu XY, Zhang R, Qian YT (2004) Large-scale growth of porous CuInS2 microspheres. Inorg Chem Commun 7(4):563–565. doi:10.1016/j.inoche.2004.02.019
Jiang Y, Qu Y, Yuan SW, Xie B, Zhang SY, Qian YT (2001) Preparation and characterization of CuInS2 nanorods and nanotubes from an elemental solvothermal reaction. J Mater Res 16(10):2805–2809. doi:10.1557/Jmr.2001.0386
Shen GZCD, Tang KB, Fang Z, Sheng J, Qian YT (2003) Polyol-mediated synthesis of porous nanocrystalline CuInS2 foam. J Cryst Growth 254:75
Shi L, Yin PQ, Wang LB, Qian YT (2012) Fabrication of single-crystalline CuInS2 nanowires array via a diethylenetriamine-thermal route. CrystEngComm 14(21):7217–7221. doi:10.1039/C2ce25368b
Baláž P (2008) Mechanochemistry in nanoscience and minerals engineering. Springer, Heidelberg
Baláž P, Achimovičová M, Baláž M, Billik P, Cherkezova-Zheleva Z, Criado JM, Delogu F, Dutková E, Gaffet E, Gotor FJ, Kumar R, Mitov I, Rojac T, Senna M, Streletskii A, Wieczorek-Ciurowa K (2013) Hallmarks of mechanochemistry: from nanoparticles to technology. Chem Soc Rev 42(18):7571–7637. doi:10.1039/C3cs35468g
Gorai S, Bhattacharya S, Liarokapis E, Lampakis D, Chaudhuri S (2005) Morphology controlled solvothermal synthesis of copper indium sulphide powder and its characterization. Mater Lett 59(28):3535–3538. doi:10.1016/j.matlet.2005.06.023
Nadica D, Abazovič DJJ, Stoiljkovič Milovan M, Mitrič Miodrag N, Ahrenkiel Scott P, Nedeljkovič Jovan M, Čomor Mirjana I (2012) Colloidal chemistry-based synthesis of quantized CuInS2/Se2 nanoparticles. J Serb Chem Soc 77(6):789–797
He JJ, Zhou WH, Li M, Hou ZL, Du YF, Wu SX (2012) One-pot route for preparation of monodisperse CuInS2 nanocrystals. Mater Lett 66(1):96–98. doi:10.1016/j.matlet.2011.08.013
Katsuhiro Nose NF, Omata Takahisa, Otsuka-Yao-Matsuo Shinya, Wataru Kato MU, Nakamura Hiroyuki, Maeda Hideaki, Hayato Kamioka AHH (2009) Photoluminescence of CuInS2-based semiconductor quantum dots; Its origin and the effect of ZnS coating. J Phys 165:012028
Landsberg PT (1991) Recombination in semiconductors. Cambridge University Press, Cambridge
Acknowledgements
The support through the Slovak Grant Agency VEGA (projects 2/0027/14, 1/0439/13, 2/0051/14) and APVV 14-0103 is gratefully acknowledged. The authors also acknowledge the support of the European Union through the CT-2011-1-REGPOT285895 AL-NANOFUN project (Advanced Laboratory for the Nano-Analysis of novel Functional materials), for the microscopy facilities sited at the Institute of Materials Science in Seville.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dutková, E., Sayagués, M.J., Briančin, J. et al. Synthesis and characterization of CuInS2 nanocrystalline semiconductor prepared by high-energy milling. J Mater Sci 51, 1978–1984 (2016). https://doi.org/10.1007/s10853-015-9507-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9507-x