Abstract
Phase equilibria in the Mg–Zn–Sr ternary system, in the composition range 0–35 at% Sr at 300 °C, were investigated using two diffusion couples and 23 alloy samples. Scanning electron microscopy equipped with an energy-dispersive spectroscope and electron probe micro-analysis were employed to analyze the constituted phases and their compositions in diffusion couples and alloy samples. X-ray diffraction (XRD) was used to analyze the crystal structure of the constituted phases in selected samples. Four new ternary compounds, Mg11−y Zn y Sr (4.7 < y < 9), Mg44Zn21Sr35, Mg20Zn62Sr18, and Mg55Zn43Sr2 were discovered in the Mg–Zn–Sr ternary system at 300 °C. In total, sixteen three-phase equilibria regions were determined in the present work. The isothermal phase diagram of the Mg–Zn–Sr ternary system, in the composition range 0–35 at% Sr, was constructed at 300 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mg-based alloys, with low density and high strength/weight ratios and near complete recycling potential, are widely used in the automotive and aeronautic industries [1]. The Mg–Zn series alloys, as the hardenable alloys, have been developed as structural materials [2] for several decades. A very good age-hardening effect can be achieved with the dissolution of Zn and the precipitates of secondary phase in the hcp (Mg) matrix with the heat treatment [3]. Unfortunately, the Mg–Zn-based alloys have the same problem as the Mg–Al series (but not the AE series), that is, poor mechanical properties at elevated temperatures which restrict their applications [4]. Recently, strontium has drawn much attention [5–9] as an important additive of Mg-based alloys for improving relatively high-temperature mechanical properties. According to Baril et al. [8], the micro-alloying of strontium in Mg-based alloys (e.g., Mg–Zn and Mg–Al series) leads to superior creep performance and excellent high-temperature mechanical properties. Moreover, Hirai et al. [9] reported that by adding Sr, high strength, and improved creep resistance could be obtained for the cast AZ91 alloys. Since Sr acts as an important micro-alloying element for Mg–Zn-based alloys, a comprehensive understanding of the phase equilibria in the Mg–Zn–Sr ternary system, especially in the Mg-rich region, is of primary importance for Mg-based alloys development.
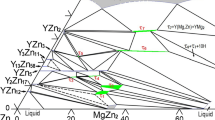
The phase diagrams of the three binary sub-systems Mg–Zn [10–13], Mg–Sr [14], and Zn–Sr [15] of the Mg–Zn–Sr ternary system have been satisfactorily investigated using experimental and thermodynamic modeling methods. The final accepted version of each binary phase diagram is shown in Fig. 1. The phase diagram of the Mg–Zn binary system includes five intermetallic compounds, Mg2Zn11, MgZn2, Mg2Zn3, Mg12Zn13, and Mg51Zn20, and two terminal solid solutions, hcp (Mg) and hcp (Zn). It is worth noting that Mg51Zn20, with minimum solid solubility, is stable only between 325 and 342 °C [16]. The phase diagram of the Mg–Sr binary system contains seven solid phases: hcp (Mg), fcc (Sr), bcc (Sr), Mg17Sr2, Mg38Sr9, Mg23Sr6, and Mg2Sr. Four stoichiometric compounds, ZnSr, Zn2Sr, Zn5Sr, and Zn13Sr, plus three terminal solid solutions, hcp (Zn), fcc (Sr), and bcc (Sr) with limited solid solubility, constitute the phase diagram of the Zn-Sr binary system. Aljarrah et al. [17] extrapolated the Mg–Zn–Sr ternary system using the CALPHAD method [18] based on the thermodynamic parameters of the three binary sub-systems. To date, no experimental data, except the study on the crystal structure and solid solubility limits of compound Mg15−x Zn x Sr3 submitted for publication by our group [19], have been reported on the phase equilibria of the Mg–Zn–Sr ternary system. All the phases of the Mg–Zn–Sr ternary system and their crystallographic information are summarized in Table 1.
In order to obtain a comprehensive understanding of the phase equilibria in the Mg–Zn–Sr ternary system for Mg-based alloys development purpose, the isothermal section of the Mg–Zn–Sr ternary system, in the Mg–Zn rich composition region (0–35 at% Sr) at 300 °C, was studied using diffusion couples and equilibrated key samples. The selection of diffusion couples and key samples was based on the extrapolated results with the three binary sub-systems. Moreover, the phase equilibria reported previously in the Mg–Zn–Ca ternary system [20] were also taken as a reference due to the similar chemical properties of Ca and Sr. The samples and diffusion couple (Mg–Mg25Zn55Sr20) discussed in our previous work [19] were reinvestigated for the phase equilibria measurements of the Mg–Zn–Sr ternary system. The target temperature of 300 °C was chosen because it corresponds to the temperature at which heat treatments are usually performed on Mg alloys. The present work is part of a wider thermodynamic database development project of Mg-X (X: Ag, Ca, In, Li, Na, Sn, Sr, and Zn)-based alloys for automotive applications [19–29].
Experimental procedure
Two solid-state diffusion couples and 32 ternary alloy samples of the Mg–Zn–Sr system were prepared using pure Mg (99.8 wt%), Zn (99.5 wt%), and Sr (99 wt%) obtained from Alfa Aesar. The nominal compositions of the end-members of the diffusion couples and other alloy samples are given in Tables 2 and 3, respectively, and shown Fig. 1. The Sr pieces were stored in oil after weighing due to the high reactivity of Sr with oxygen. Before melting, each Sr piece was washed carefully with 99 wt% ethanol to remove the oil. In order to minimize the interaction of the samples with the crucibles, cubic-shaped crucibles were made using Ta foil (99.5 wt% purity, 0.15 mm thickness). All samples were re-melted at least 3 times in the induction furnace under argon in order to obtain a homogeneous alloy. To prepare solid-state diffusion couples, the contacting surfaces of the alloy pieces were ground with 1200 grit SiC paper and polished using a 1 μm oil-based diamond suspension with 99 % pure ethanol as lubricant. The end-members were gently pressed and clamped with a steel ring. Diffusion couples and alloy samples were then sealed into quartz capsules under argon and equilibrated at 300 °C for 21 and 35 days, respectively. Quenching was carried out in water without breaking the quartz tube to avoid any reaction of the samples with water.
Phase relationships and constitution of the alloy samples were analyzed using electron probe micro-analysis (EPMA) with wavelength dispersive X-ray spectrometry (WDS) and scanning electron microscopy (SEM) equipped with an energy-dispersive spectroscope (EDS). An accelerating voltage of 15 kV was used for the EPMA/EDS analysis with a 20 nA beam current, a spot size of 2 µm and counting times of 20 s on peaks and 10 s on backgrounds. Raw data were reduced with the phi-rho-Z (PRZ) correction using pure Mg, Zn metal, and SrO standards. Phase relationships and constitutions of the diffusion couples were determined using SEM/EDS. An accelerating voltage of 20 kV was used with a spot size of 3 µm and counting times of 50 s. Five points in different areas of each target phase were measured using EPMA/EDS to obtain an average value of the phase composition.
Crystal structures of the phases present in the annealed samples were identified by X-ray diffraction (XRD). XRD patterns were obtained with a PAN analytical X’pert Pro powder X-ray diffractometer using CuKα radiation at 45 kV and 40 mA. The patterns were acquired from 20–120° (2θ) with a 0.02 step size. The collected patterns were analyzed with the X’Pert HighScore plus Rietveld analysis software in combination with the Pearson’s crystal database [30]. Si was used as an internal calibration standard to enable correcting the zero shift and specimen surface displacement, which are the most serious systematic errors in X-ray powder diffraction patterns.
Results and discussion
Diffusion couples results
In order to obtain general information on the equilibrium phase relationships in the Mg–Zn–Sr ternary system, two diffusion couples, D1 (Zn–Mg75Zn12.5Sr12.5) and D2 (Mg–Mg25Zn55Sr20), as shown in Fig. 1, were prepared and investigated.
Back-scattered electron (BSE) images obtained by SEM of the diffusion couple D1, with gradually increased magnification of the area of interest, are shown in Fig. 2. During the heat treatment, extensive inter-diffusion of Mg, Zn, and Sr took place, allowing various equilibrated phases to form. As shown in Fig. 2a, several diffusion layers were formed in the diffusion couple D1 after the heat treatment. The new compound designated as Mg15−x Zn x Sr3 (with detailed crystal structure information given in Ref. [19] ), in equilibrium with the Mg17Sr2 phase, was also observed in the end-member alloy Mg75Zn12.5Sr12.5 (at%), as shown in Fig. 2b. The equilibrium phase compositions of Mg15−x Zn x Sr3 and Mg17Sr2 in the diffusion couple D1, obtained using EDS, are listed in Table 2. In the diffusion couple D1, a second new ternary compound (Mg11−y Zn y Sr), designated as IMI, IM3 and IM5 in our previous work [19], was observed as shown in Fig. 2c–e. According to the EDS analysis of the diffusion couple D1, the solid solubility of this compound ranges from about 42–81 at% Zn with a constant value of about 9 at% Sr. This compound was thus designated as IM1 (Mg11−y Zn y Sr, 4.7 < y < 9) in the present work. The element compositions of Mg11−y Zn y Sr and its equilibrated phases, obtained using EDS, are listed in Table 2. Taking advantage of the local equilibrium at the interfaces formed between the phases, the sequence of the phases formed and the diffusion path in the diffusion couple D1 could be deemed to be Mg15−x Zn x Sr3 + Mg17Sr2 ↔ Mg11−y Zn y Sr + MgZn2 ↔ Zn13Sr ↔ Zn (hcp). The diffusion path of the diffusion couple D1 is shown in Fig. 3. Note that the diffusion interface of hcp (Zn) and Zn13Sr was lost due to inevitable cracks which occurred during the quenching and polishing processes.
The diffusion couple D2 has been investigated to analyze the solid solubility of the new compound Mg11Zn4Sr3 reported in our previous work [19]. In this section, the phase equilibrium information obtained from the diffusion couple D2 was reinvestigated and further discussed. BSE images of the diffusion couple D2 are shown in the Fig. 4. Four diffusion layers, viz., Mg15−x Zn x Sr3, Mg11−y Zn y Sr, Mg17Sr2, and hcp (Mg), were observed as shown in Fig. 4a. The two-phase equilibrated microstructure Zn2Sr + Mg15−x Zn x Sr3 was observed in the end-member of Mg25Zn55Sr20 alloy as shown in Fig. 4b. The EDS analytical results on the constituted phase compositions are listed in Table 2. The solid solubility of Mg in Zn2Sr was measured to be about 1.3 at%. However, according to the composition value of 17.8 at% Sr obtained in the constituted phase of Mg15−x Zn x Sr3, the two phases Zn2Sr and Mg15−x Zn x Sr3 in the end-member of Mg25Zn55Sr20 alloy need more time to reach complete equilibrium. The phase Mg11−y Zn y Sr, found in the diffusion couple D1, was also observed in the diffusion couple D2 in the three-phase equilibrated microstructure Mg15−x Zn x Sr3 + Mg11−y Zn y Sr + Mg17Sr2 (see Fig. 4a, c). The equilibrium composition of each phase of Mg15−x Zn x Sr3, Mg11−y Zn y Sr, and Mg17Sr2, in the three-phase assemblage Mg15−x Zn x Sr3 + Mg11−y Zn y Sr + Mg17Sr2, was measured at the position where all the three phases existed (marked as triangles in Fig. 4c). Average values were obtained with more than 5 different measurement positions, which were summarized in Table 2.
The sequence of the diffusion paths in the diffusion couple D2 was determined as follows Mg15−x Zn x Sr3 ↔ Mg11−y Zn y Sr + Mg15−x Zn x Sr3 + Mg17Sr2 ↔ Mg17Sr2 + Mg (hcp) ↔ Mg (hcp) as shown in Figs. 3 and 4. The EDS line scan was used to determine the composition profile of phases formed as shown in Fig. 4d. According to the acquired line-scan results, the solid solubility limit of Zn in Mg15−x Zn x Sr3 was found to be about 29–37 at% with substitution of Mg at a constant Sr content of about 16.7 at%. Equilibrium compositions of the constituted phases, obtained from the diffusion couple D2 using EDS, are summarized in Table 2.
According to the analytical results from the two diffusion couples D1 and D2, a new compound IM1 (Mg11−y Zn y Sr, 4.7 < y < 9) was observed in the Mg–Zn–Sr ternary system at 300 °C. More accurate solubilities were further confirmed and measured using the following alloy samples.
Alloy samples results
Based on the phase equilibria relationships obtained from diffusion couples D1 and D2, 23 samples (A1–A23) were selected and prepared in order to confirm the phase relationships, and to construct an isothermal section of the Mg–Zn–Sr ternary system at 300 °C.
BSE images obtained from typical Mg–Zn–Sr ternary key samples are shown in Fig. 5a–i. The new ternary compound Mg11−y Zn y Sr (4.7 < y < 9) observed in diffusion couples D1 and D2 was confirmed in the key samples A8–A10, A12, and A15–A20 after annealing at 300 °C. In sample A10 (Mg3Zn87Sr10), the three-phase equilibrium Zn5Sr + Zn13Sr + Mg11−y Zn y Sr was observed as shown in Fig. 5a. According to the EPMA results of the constituted phase compositions in the key sample A10, the maximum solid solubility limit of Zn in the new phase Mg11−y Zn y Sr was found to be 84.2 at%. A three-phase equilibrated microstructure MgZn2 + Zn13Sr + Mg11−y Zn y Sr was observed in the sample A12 (Mg16Zn80Sr4) as shown in Fig. 5b. A value close to the maximum solid solubility limit of Zn in phase Mg11−y Zn y Sr was found to be 83.7 at% in sample A12. As shown in Fig. 5c, another three-phase equilibrium hcp (Mg) + Mg17Sr2 + Mg11−y Zn y Sr was found in the key sample A19 (Mg80Zn15Sr5). Deservedly, the minimum solid solubility limit of Zn in Mg11−y Zn y Sr, in the Mg-rich region, was found to be 42.0 at% by EPMA.
The two-phase equilibrium Mg11−y Zn y Sr + Mg15−x Zn x Sr3 was observed in the alloy sample A8 (Mg35Zn55Sr10) as shown in Fig. 5d. This confirmed that Mg11−y Zn y Sr is a single phase with a large solid solubility composition range of Zn without miscibility gap. The constituted phases in sample A8 were also confirmed using XRD as shown in Fig. 6a. Due to the complex crystal structure and atoms occupancy of Mg11−y Zn y Sr, these XRD results will be discussed in a coming paper.
The solubility and equilibria of compound Mg15−x Zn x Sr3 were re-analyzed using samples A1–A8, A13, A15, and A20–A23 based on the results from our previous work [19]. In the three-phase equilibrium Mg15−x Zn x Sr3 + Mg17Sr2 + Mg38Sr9 observed in the key sample A1 (Mg83Zn1Sr16) (as shown in Fig. 5e), the minimum solid solubility limit of Zn in the compound Mg15−x Zn x Sr3 was found to be 1.3 at%. The phase constitutions of key sample A1 was also confirmed by the XRD results. The X-ray diffraction pattern obtained from sample A1 is depicted in Fig. 6b, where the XRD peaks of Mg15−x Zn x Sr3, Mg17Sr2, and Mg38Sr9 can be observed clearly. A BSE image of sample A15 (Mg20Zn65Sr15) is shown in Fig. 5f, where the three-phase equilibrium Mg15−x Zn x Sr3 + Mg11−y Zn y Sr + Mg20Zn62Sr18 can be seen. The maximum solid solubility of Zn in Mg15−x Zn x Sr3 is thus confirmed to be 58.8 at%. Moreover, a third compound with about 18 at% Sr, designated as IM5 (Mg20Zn62Sr18) in our previous work [19], was confirmed in sample A15 (see Fig. 5f). This compound was also observed in samples A9, A14, and A21 in the three-phase equilibria Mg20Zn62Sr18 + Zn5Sr +Mg11−y Zn y Sr, Mg20Zn62Sr18 + Zn2Sr + Zn5Sr, and Mg20Zn62Sr18 + Mg15−x Zn x Sr3 + Zn2Sr, respectively. The element composition of IM5 obtained using EPMA/EDS tends to give a constant value of Mg20Zn62Sr18; the formula “Mg20Zn62Sr18” was thus employed to designate IM5 in the present work. It is worth noting that more research work is needed to confirm the solid solubility and crystal structure of IM5 (Mg20Zn62Sr18).
A fourth new compound, with a formula designated as “Mg55Zn43Sr2 (IM6)” based on its solid solubility composition range, was found in the alloy sample A17 (Mg49Zn47Sr4), as shown in Fig. 5g, where the three-phase equilibrium Mg55Zn43Sr2 + Mg12Zn13 + Mg11−y Zn y Sr was observed. The solubility limit of Sr was measured to be about 2 at%, which is similar to the Ca content in the Mg55Zn43Ca2 compound reported by Zhang et al. [20]. The compound Mg55Zn43Sr2 was also observed in the sample A18 (Mg60Zn35Sr5) in equilibrium with hcp (Mg) + Mg11−y Zn y Sr as listed in Table 3.
In the sample A23 (Mg50Zn25Sr25), a fifth new compound, designated as IM2 in our previous work [19], was confirmed; it is in equilibrium with Mg2Sr + Mg15−x Zn x Sr3 (see Fig. 5h). This compound IM2 was also found in A13 (Mg25Zn50Sr25) with the three-phase equilibrium Zn2Sr + IM2 + Mg15−x Zn x Sr3 as shown in Fig. 5i. EDS/EPMA results show that the element composition in IM2 is close to the constant value of Mg44Zn21Sr35. Consequently, the formula of IM2 was designated as “Mg44Zn21Sr35” in the present work.
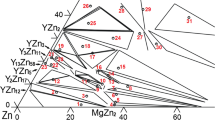
The phase equilibria of the Mg–Zn–Sr ternary system in the composition range 0–35 at% Sr at 300 °C was obtained using alloy samples as shown in Fig. 7. Each of the determined three-phase equilibrium was labeled with different colors for easy reading purpose. All the compositions of the constituent phases of the equilibrated Mg–Zn–Sr ternary samples were determined by EPMA/EDS and are listed in the Table 3. Samples labelled with a star “*” were analyzed using EDS and corrected using EPMA standards. The phase constitutions and compositions of A1–A8 and A15 were reinvestigated based on the results obtained in our previous work [19]. Both phase equilibria relationships and compositions, obtained using equilibrated alloys and diffusion couple techniques, are in good agreement.
Isothermal section
The isothermal section of the Mg–Zn–Sr ternary system at 300 °C, obtained using alloy samples and diffusion couples, is shown in Fig. 8. Sixteen three-phase domains, lying in the composition range 0–35 at% Sr, have been accurately determined in the present work. Four new ternary compounds, Mg11−y Zn y Sr (IM, 4.7 < y < 9), Mg44Zn21Sr35 (IM2), Mg20Zn62Sr18 (IM5), and Mg55Zn43Sr2 (IM6) along with the previous reported compound Mg15−x Zn x Sr3, were found in the present work. The solid solubility limits of Zn in Mg15−x Zn x Sr3 ranges between 1.3 and 58.8 at% with a constant Sr content of 16.7 at% [19]. The solid solubility limits of Zn (substituting to Mg) in Mg11−y Zn y Sr ranges between 42.0 and 84.2 at% at a nearly constant Sr content of about 8 at%. According to the present results obtained from alloy samples as listed in Table 3, the compounds Mg44Zn21Sr35, Mg20Zn62Sr18, and Mg55Zn43Sr2 have limited solid solubility limits. They are treated as stoichiometric compounds by the present authors. The maximum solid solubility limits of Zn in Mg17Sr2 and Mg23Sr6 were measured to be 20.2 and 12.4 at% with Zn substitution by Mg at constant Sr contents of 10.7 and 20.0 at%, respectively. The solubility limits of Sr in the hcp (Mg), hcp (Zn), and Mg–Zn binary terminal compounds are inferior to 1.5 at%. The solid solubility limits of Mg in Zn2Sr and Zn5Sr were found to be about 1.8 and 2.6 at%, respectively. The solubility limit of Mg in the Zn13Sr compound is negligible (around 0.1 at%).
There are still four undetermined three-phase assemblages in the Mg-Zn rich region: Mg38Sr9 + Mg23Sr6 + Mg15−x Zn x Sr3, Mg2Zn3 + Mg11−y Zn y Sr + MgZn2, hcp (Zn) + Mg2Zn11 + Zn13Sr, and hcp (Mg) + Mg55Zn43Sr2 + Mg12Zn13. They are indicated with dashed lines in Fig. 8. Phase equilibria in the Sr-rich region (above 35 at%) were proposed as inferred by the authors based on the results reported for the Mg–Zn–Ca ternary system [20]. Further experiment will be necessary to verify them. Crystal structure analysis and thermal stability of the ternary compounds Mg11−y Zn y Sr, Mg44Zn21Sr35, Mg20Zn62Sr18, and Mg55Zn43Sr2 are still needed.
Conclusions
The isothermal section of the Mg–Zn–Sr ternary system at 300 °C, in the composition region 0–35 at% Sr, has been investigated using diffusion couples and key samples. The ternary compounds Mg11−y Zn y Sr (IM1, 4.7 < y < 9), Mg44Zn21Sr35 (IM2), Mg20Zn62Sr18 (IM5), and Mg55Zn43Sr2 (IM6) were found and their solid solubility limits were determined. Sixteen three-phase domains were determined. The extended solid solubility limits of all the binary compounds, except ZnSr, were also determined in the Mg–Zn–Sr ternary system. The isothermal section of the Mg–Zn–Sr system at 300 °C was drawn. The remaining four three-phase domains in the Mg–Zn rich region were proposed as follows: Mg38Sr9 + Mg23Sr6 + Mg15−x Zn x Sr3, Mg2Zn3 + Mg11−y Zn y Sr + MgZn2, hcp (Zn) + Mg2Zn11 + Zn13Sr, and hcp (Mg) + Mg55Zn43Sr2 + Mg12Zn13. Phase equilibria in the Sr-rich region (above 35 at%) were proposed as inferred by the authors.
References
Polmear I (1994) Magnesium alloys and applications. Mater Sci Tech Lond 10(1):1–16
Avedesian MM, Baker H (1999) ASM specialty handbook: magnesium and magnesium alloys, vol 274. ASM International
Gao X, Nie J (2007) Characterization of strengthening precipitate phases in a Mg–Zn alloy. Scripta Mater 56(8):645–648
Kainer KU, Kaiser F (2003) Magnesium alloys and technology. Wiley Online Library
Aljarrah M, Medraj M (2008) Thermodynamic modelling of the Mg–Ca, Mg–Sr, Ca–Sr and Mg–Ca–Sr systems using the modified quasichemical model. Calphad 32(2):240–251
Janz A, Schmid-Fetzer R (2009) Thermodynamics and constitution of Mg–Al–Ca–Sr–Mn alloys: part I. experimental investigation and thermodynamic modeling of subsystems Mg–Ca–Sr and Al–Ca–Sr. J Phase Equilib Diff 30(2):146–156
Liu H, Chen Y, Zhao H, Wei S, Gao W (2010) Effects of strontium on microstructure and mechanical properties of as-cast Mg-5wt.% Sn alloy. J Alloy Compd 504(2):345–350
Baril E, Labelle P, Pekguleryuz M (2003) Elevated temperature Mg-Al-Sr: creep resistance, mechanical properties, and microstructure. J Min Met Mater Soci 55(11):34–39
Hirai K, Somekawa H, Takigawa Y, Higashi K (2005) Effects of Ca and Sr addition on mechanical properties of a cast AZ91 magnesium alloy at room and elevated temperature. Mater Sci Eng A 403(1):276–280
Ghosh P, Mezbahul-Islam MD, Medraj M (2012) Critical assessment and thermodynamic modeling of Mg–Zn, Mg–Sn, Sn–Zn and Mg–Sn–Zn system. Calphad 36:28–43
Meng F, Wang J, Liu L, Jin Z (2010) Thermodynamic modeling of the Mg-Sn-Zn ternary system. J Alloy Compd 508(2):570–581
Wang J, Hudon P, Kevorkov D, Chartrand P, Jung I-H, Medraj M (2014) Experimental and thermodynamic study of the Mg–Sn–In–Zn quaternary system. J Alloy Compd 588:75–95
Agarwal R, Fries SG, Lukas HL, Petzow G, Sommer F, Chart TG, Effenberg G (1992) The Mg-Zn system. Z Metallkd 83:216–223
Nayeb-Hashemi A, Clark J (1986) The Mg–Sr (magnesium-strontium) system. J Phase Equilib 7(2):149–156
Bruzzone G, Merlo F (1983) The Sr-Zn system. J Less-common Met 92(1):75–79
Clark J, Rhines F (1957) Central region of the Mg–Zn phase diagram. Trans Am Inst Min Metall Eng 209:425–430
Aljarrah M, Aghaulor U, Medraj M (2007) Thermodynamic assessment of the Mg–Zn–Sr system. Intermetallics 15(2):93–97
Dinsdale AT (1991) SGTE data for pure elements. Calphad 15:317–425
Wang J, Zhang Y-N, Hudon P, Jung I-H, Chartrand P, Medraj M (2015) Experimental study of the crystal structure of the Mg15−xZnxSr3 ternary solid solution in the Mg–Zn–Sr system at 300 ºC. J Mater Des 86:305–315
Zhang Y-N, Kevorkov D, Liu XD, Bridier F, Chartrand P, Medraj M (2012) Homogeneity range and crystal structure of the Ca2Mg5Zn13 compound. J Alloy Compd 523:75–82
Wang J, Jiajia H, Chartrand P, Jung I-H (2014) Thermodynamic optimizations on the binary Li-Sn system and ternary Mg–Sn–Li system. Calphad 47:100–113
Wang J, Hudon P, Kevorkov D, Chartrand P, Jung I-H, Medraj M (2014) Experimental and thermodynamic study of the Mg–Sn–In–Zn quaternary system. J Alloy Compd 588:75–95
Wang J, Miao N, Chartrand P, Jung I-H (2013) Thermodynamic evaluation and optimization of the (Na + X) binary systems (X = Ag, Ca, In, Sn, Zn) using combined Calphad and first-principles methods of calculation. J Chem Thermodyn 66:22–33
Wang J, Hudon P, Kevorkov D, Chartrand P, Jung I-H, Medraj M (2014) Thermodynamic and experimental study of the Mg-Sn-Ag-In quaternary system. J Phase Equilib Diff 35(3):284–313
Zhang Y-N, Kevorkov D, Bridier F, Medraj M (2011) Experimental study of the Ca–Mg–Zn system using diffusion couples and key alloys. Sci Technol Adv Mater 12(2):025003
Zhang Y-N, Kevorkov D, Li J, Essadiqi E, Medraj M (2010) Determination of the solubility range and crystal structure of the Mg-rich ternary compound in the Ca–Mg–Zn system. Intermetallics 18(12):2404–2411
Zhang Y-N, Rocher G, Briccoli B, Kevorkov D, Liu X, Altounian Z, Medraj M (2013) Crystallization characteristics of the Mg-rich metallic glasses in the Ca–Mg–Zn system. J Alloys Compd 552:88–97
Wang J, Zhang Y-N, Hudon P, Jung I-H, Medraj M, Chartrand P (2015) Experimental study on the phase equilibria of Mg-Zn-Ag ternary system at 300°C. J Alloys Compd 639:593–601
Wang J, Jung I-H, Chartrand P (2015) Thermodynamic modeling on Ag-(Ca, Li, Zn) and Ca-(In, Li) binary systems. Calphad 50:68–81
Villars P, Cenzual K (2007) Pearson’s crystal data, crystal structure database for inorganic compounds. ASM International, Materials Park
Acknowledgements
Financial support from General Motors of Canada Ltd., and the Natural Sciences and Engineering Research Council of Canada through the CRD grant program is gratefully acknowledged. The support in the experimental part from Mr. Tian Wang, Mr. Xin Zhang, and Dr. Dmytro Kevorkov from Concordia University, and Mr. Lang Shi from McGill University is acknowledged by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, J., Zhang, YN., Hudon, P. et al. Experimental determination of the phase equilibria in the Mg–Zn–Sr ternary system. J Mater Sci 50, 7636–7646 (2015). https://doi.org/10.1007/s10853-015-9326-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9326-0