Abstract
In this study, we explored the feasibility of fabrication bioactive mesoporous calcium silicate/calcium phosphate cements (MCS/CPC) scaffolds with high mechanical strength by Freeform Fabrication System with Micro-Droplet Jetting. After preparation of ordered mesoporous calcium silicate (MCS) powder, ready-to-use MCS/CPC paste was formed by mixing calcium phosphate cement (CPC) powder and MCS powder with the binder polyvinyl alcohol (PVA) aqueous solution at a certain ratio of powder to liquid. MCS/CPC scaffolds with various architectures, pore sizes, and interconnectivity were then directly printed at room temperature using MCS/CPC paste. The mechanical strength, apatite formation, degradation rate, and cytocompatibility of the composite scaffolds were systematically investigated. The results showed that MCS/CPC paste exhibited outstanding printability to form MCS/CPC scaffolds. The hybrid MCS/CPC scaffolds with predefined pore size of 350 μm showed fast degradation rate, high mechanical strength, and good cytocompatibility. It was indicated that the hybrid MCS/CPC scaffolds might be a promising candidate for critical bone defect repair.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Scaffold-guided bone tissue engineering aims to rebuild the configuration of defected bone tissue and its physiological function for improved bone regeneration [1]. Degradable scaffolds with 3D macroporous structure have received a great attention recently. Due to its good biocompatibility and osteoconductivity to form bone bonding with host bone, Calcium phosphate cements (CPCs) have been paid special attention for many years [2, 3]. Our group developed one novel CPC product with unique advantages of self-setting and easily-shaped capability [4]. Under physiological conditions, the CPC is able to set and harden to form hydroxyapatite (HA) after being mixed with aqueous solution [5, 6]. The mechanical strength of CPC is improved due to the formation of HA [6]. However, HA formed with a relatively low dissolution rate would lead to the low degradation, which might hinder the fast formation and restoration of new bone after being implanted in human body [7]. For certain clinical applications, more rapid resorption by new bone is desirable. Therefore, it is necessary to develop new CPC-based biomaterial with faster degradation rate.
The introduction of macroporous structure and modifying biomaterials are two generally accepted ways to obtain CPC-based scaffolds with enhanced properties. It is founded that macropores in CPC implant may lead to more rapid resorption and concomitant osseointegration of the implant [8]. As a matter of fact, three-dimensional (3D) macroporous structure of scaffold is a critical factor affecting the bone regeneration. It should not only provide environment for cell interaction, ingrowth, and diffusion of nutrients, but also structural support to resist external stress and maintain the outer shape [9, 10].
Macroporous CPC scaffolds were prepared by conventional methods including particle leaching, freeze drying, and gas foaming [11–14]. However, these methods have their drawbacks such as low mechanical strength, long fabrication periods, poor repeatability, irregularly shaped pores, and insufficient interconnectivity of pores, which makes it hard to simulate the inner microstructure of bone. Furthermore, most of these methods bear restrictions on shape control.
Rapid prototyping (RP) has been developed to prepare porous scaffolds in recent years [15]. The significant advantages are to manufacture scaffolds with complex structures such as defect site matched outer shape, as well as designed internal channel network to mimic bone structures [16–23]. 3D inject printing, one of the most important RP techniques, has been used to fabricate calcium phosphate cement scaffolds directly from powder materials [24–26]. Although scaffolds with controllable porous structure can be successfully fabricated with this technique, it is difficult to removing the entrapped powder from the porous structure. Moreover, mechanical strength of these scaffolds is low. Scaffolds constantly require additional hardening process such as immersing in a diluted solution of phosphoric acid or sintering. It is well known that low strength along with brittleness makes the porous scaffolds difficult to handle and even undermine their biologic performances. Furthermore, post-processing might limit the fabrication of scaffolds with drugs or cells at one time. 3D extrusion-based printing is an emerging technique used for creating artificial tissue scaffolds [27–29]. Recently, scaffolds with controllable pore structure and smooth surface were directly fabricated without complicated post-treatment, which made it possible for the introduction of proteins, drugs, and cells in the scaffolds during the fabrication process [23].
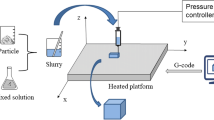
In this study, freeform fabrication system with micro-droplet jetting (FFS), a fabrication system based on 3D extrusion-based printing was used to prepare porous scaffolds. It worked with a linear stepper motor by driving a piston to extrude printing paste. Printing paste was formed by mixing CPC-based powders with polyvinyl alcohol aqueous solution as binder sufficiently. CPC-based scaffolds with macroporous structure could be fabricated by FFS at room temperature. However, short setting time of CPC reminded us that close attention should be paid to the fluidity and extrusion performance of the paste.
CS ceramics have received significant attention for application in bone regeneration in the past few years. Our previous study revealed that the addition of 10–20 % low crystalline CS into CPC could effectively improve the bioactivity as well and degradability of CPC [30, 31]. In addition, the setting time of composite cement was markedly extended [31]. Silica-based ordered mesoporous matrices have been widely applied in delivery systems due to highly ordered mesoporous structure and large surface area, which tended to be more bioactive and bioresorbable [32]. It motivated us to introduce the mesoporous calcium silicate (MCS) to the CPC paste to improve the biodegradation and the printing property.
To the best of our knowledge, there has been no previous report describing the fabrication of mesoporous calcium silicate/calcium phosphate cement scaffolds with interconnected macroporosity by FFS. The objective of this study was to develop hybrid bioactive MCS/CPC scaffolds with both porous structure and high mechanical strength. First, MCS powder was synthesized and mixed with CPC powder as printing material to fabricate scaffolds. Scaffolds with diverse shapes as well as internal channel networks were designed to verify the printability of MCS/CPC paste. Secondly, MCS/CPC scaffolds with predefined pore size of 350 μm were chosen for further study, which included mechanical strength, bioactivity, degradation rate, and cytocompatibility.
Materials and methods
Preparation and characterization of CPC and MCS powders
The CPC powder used in this experiment was prepared in our laboratory, and the preparation methods could be obtained from the relevant literature [5]. TECP was synthesized by a solid-to-solid reaction between hydroxyapatite and calcium carbonate at 1500 °C for 8 h. Dicalcium phosphate dihydrate (DCPD) was prepared from (NH4)2HPO4 and Ca(NO3)2 in the acidic solution, and DCPA was obtained by removing the crystal water in DCPD at 120 °C for 10 h. The optimum heat treatment temperature was 120–140 °C. The CPC powder was composed of TECP and DCPA in an equivalent molar ratio.
MSC powder was synthesized by sol–gel method using nonionic block copolymer EO20PO70EO20 (Mw 5800, Sigma-Aldrich, Germany) as a structure directing agent and tetraethylorthosilicate (TEOS, Sigma-Aldrich, Germany) as silica source. In brief, 4 g triblock copolymer was mixed with 150 mL HCl (1.6 mol L−1) in a beaker, and then TEOS was added drop by drop to hydrolyze for 30 min (mol ratio: TEOS/H2O/HCl = 1:4:0.08). Subsequently, 5.2 g Ca (NO3)2·4H2O was added to the above reaction mixture and the reactants were stirred for 5 h at room temperature. After the reaction, the solution was heated under 100 °C for another 48 h. The obtained white solid was filtered and washed repeatedly with deionized water, and then dispersed in 150 mL deionized water. Then 5.2 g of Ca (NO3)2·4H2O was added to the dispersion and stirred for 2 h. After drying overnight, the powder was sintered in a muffle furnace at 600 °C for 6 h at a heating rate of 1 °C min−1 to obtain the MCS for further experiments.
The formation of MCS was observed by scanning electron microscopy (SEM, JSM-6360LV, JEOL, Japan). The ordered mesoporous structure of MCS was confirmed by small-angle X-ray diffraction (SAXRD, Rigaku D/max 2550VB/PC, Japan) and transmission electron microscopy (TEM, JEM2010, Japan). The phase transition of MCS was characterized by wide-angle X-ray diffraction (WAXD, D/max 2550 V, Japan).
Fabrication of CPC and MCS/CPC scaffolds
After CPC powder was grinded with pot mill, CPC and MCS powders were screened with a 500 mesh sieve separately. The CPC powder was then mixed with 20 wt% MCS to form the blended MCS/CPC powder. A 10 wt% PVA (Mw 98000; Sigma-Aldrich, USA) aqueous solution was used as a binder. Printable CPC and MCS/CPC pastes were prepared by mixing the powders with the binder at the fixed powder to liquid (P/L) ratio of 2.5 and 2 g mL−1, respectively.
Printable paste was first fed into the stainless steel syringe of FFS (Fochif Co. Ltd., China) at room temperature, and then extruded through the small nozzle with the inner diameter of 400 μm. The extrudate was printed layer by layer according to the predefined pattern with a layer thickness of 0.40 mm to produce porous structure. The scaffolds were carefully printed by moving the X–Y positioning platform at 3 mm/s, and then hardened at 37 °C in a 100 % humidity chamber for 72 h.
Printable time of CPC and MCS/CPC paste
CPC and MCS/CPC pastes were formed by mixing CPC and MCS/CPC powder with the binder at the fixed P/L ratio mentioned above, respectively. The printable time was determined to be the time from loading the pastes into the FFS to small nozzle being blocked by setting product. Each experiment was performed in triplicate.
Mechanical strength of scaffolds
CPC and MCS/CPC scaffolds (10 × 10 × 10 mm3) with predefined pore size of 350 μm were fabricated by FFS. After hardening at 37 °C in a 100 % humidity chamber for 72 h, the compressive strength of the CPC and MCS/CPC scaffolds was measured at a loading rate of 1 mm min−1 using a universal testing machine (AG-2000A, Shimadzu Autograph, Shimadzu Co. Ltd., Japan). Three replicates were carried out for each group.
Degradation of MCS/CPC scaffolds in vitro
The degradation behaviors of MCS/CPC scaffolds with pore size of 350 μm (10 × 10 × 2 mm3) were measured in 0.05 M Tris-HCl solution (pH 7.4) at 37 °C in a shaking water bath for 3, 7, 14, 21, 28, 35, and 42 days. CPC scaffolds were chosen as control. The solution was refreshed every week. The mass ratio of the scaffold to solution was 0.5 g g−1. After the set soaking time, the specimens were removed, rinsed with distilled water, and dried in an over at 100 °C for 2 h. The final weight of each specimen was measured. The weight loss of the scaffolds was calculated as follows:
where W 0 is the initial dry weight and W t is the final dry weight of the scaffolds. Each experiment was performed in triplicate.
In vitro mineralization
Simulated buffered saline (SBF) was prepared according to the procedure described by Kokubo [33]. The scaffolds (10 × 10 × 2 mm3) were soaked in SBF at 37 °C for 7 days. The ratio of solution to scaffold mass was 200 mL g−1. Three samples were tested for replicate experiments. The scaffolds before and after being immersed in SBF were evaluated by scanning electron microscopy (SEM, JSM-6360LV, JEOL, Japan), energy dispersive spectroscopy (EDX, Falcon, USA), and wide-angle X-ray diffraction (WAXD, D/max 2550 V, Japan) for morphological and compositional analysis, respectively.
Cell viability
CPC and MCS/CPC scaffolds (10 × 10 × 2 mm3) were first put in each well of the 24-well culture plate. C2C12 cells were then seeded onto the scaffolds at a density of 2 × 104 cells per scaffold, followed by static incubation at 37 °C and 100 % humidity with 5 % CO2 in Dulbecco’s modified Eagle’s medium–bovine fetal serum (DMEM). The medium was changed every 2 days. The cell viability was determined using a methyl thiazolyl tetrazolium (MTT) assay. After culturing for 1, 3, and 7 days, 100 μL of MTT solution was added into each well in the plate. The plate was then incubated for further 4 h. The supernatant from each well was then removed and 200 μl of dimethyl sulfoxide was added. After shaking for 10 min, the optical density (OD) of the chromophore was measured at 492 nm using an enzyme-linked immuno adsorbent assay plate reader. Cells cultured directly on 24-well plate were used as control. Results were reported as the percentage ratio of ODsample/ODcontrol × 100 % (n = 3).
Cell morphology
CPC and MCS/CPC scaffolds (10 × 10 × 2 mm3) were first put in each well of the 24-well culture plate. C2C12 cells were then seeded onto the scaffolds at a density of 2 × 104 cells per scaffold. After static incubated in DMEM at 37 °C and 100 % humidity with 5 % CO2 for 72 h, samples were washed with phosphate-buffered saline (PBS) twice and fixed with formalin solution (3.7 % formaldehyde in PBS) for 15 min. The fixed cells were washed with PBS twice, and then stained with FITC-Phalloidin (Sigma, St. Louis, USA) for 40 min. 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI, Beyotime Biotech, Jiangsu, China) solution was added to stain cell nuclei for 10 min. The specimens were observed using confocal laser scanning microscope (CLSM, Nikon, Japan).
Results
Characterization of MCS
The SEM micrographs of MCS are shown in Fig. 1. These micrographs confirmed the formation of a well ordered structure for MCS consisting of rod-shaped particles of 1500–2000 nm in length and 450–550 nm in diameter.
The wide-angle X-ray diffraction patterns in Fig. 2a show a relatively wide peak around 2θ = 30°, which indicate the MCS powders synthesized are amorphous. The small-angle XRD was applied to monitor the mesoporous structure of MCS. As illustrated in Fig. 2b, distinct diffraction peaks indexed to (100) reflections and weak signal peaks indexed to (110) reflections could be clearly identified, indicating the ordered mesoscopic symmetry of MCS.
TEM images were taken to directly observe the morphology of the synthesized samples. The MCS (Fig. 3) exhibited a uniform distribution of mesopores, which was in accordance with the result of SAXD.
Printability of CPC and MCS/CPC paste
The printable time of CPC paste was about 10 min. It was observed that CPC paste turned into solid mass in the fabrication system thereafter, which made printing the porous scaffold difficult. With the addition of MCS, the printable time of MCS/CPC extended to about 120 min. The longer printable time made MCS/CPC paste extruded continuously and homogeneously. Figure 4 illustrates the MCS/CPC scaffolds with various architectures and pore structures. The inner pore shape of the MCS/CPC scaffold was first controlled by adjusting the angle of the process pattern. Figure 4 (a1–a4) presented diverse pore structures of MCS/CPC scaffolds with −15°/15°, −30°/30°, −45°/45°, and −45°/45°/0°/90° patterns in X–Y direction. It could be clearly seen that the macropores in the scaffolds were uniform and completely open. In addition, scaffolds with different pore structures in Z direction were prepared precisely (Fig. 4b1–b4). Tight bonding among different layers was observed in scaffolds. To meet a wide range of individual needs, MCS/CPC scaffolds with different outer architectures were also designed and fabricated successfully (Fig. 4c1–c8).
Mechanical properties of scaffolds
The compressive stress–strain curves of CPC and MCS/CPC scaffolds are shown in Fig. 5. CPC and MCS/CPC scaffolds with the same interconnected pore structure showed the maximal compressive strength of 18.5 and 17.0 MPa, respectively. The result suggested that the introduction of MCS did not decrease the mechanical strength of the scaffolds greatly.
Degradation of scaffolds
Figure 6 presents the weight loss of CPC and MCS/CPC soaked in Tris-HCl solution with time. It was clear that both CPC and MCS/CPC continued to degrade over the entire incubation period. The MCS/CPC scaffolds had a relatively high weight loss, reaching up to 37 % after 42 days. In contrast, CPC scaffolds showed much lower weight loss, only 14 % at the same time. The results indicated that the addition of MCS could accelerate the degradation of scaffolds.
In vitro mineralization
Figure 7 shows SEM images of the MCS/CPC scaffold before (Fig. 7a) and after (Fig. 7b) SBF immersion. After being immersed in SBF for 7 days, plate-like crystallized apatite particles formed large aggregates on the surface of the scaffolds. The EDS analysis showed that Ca and P peaks increased while Si peak disappeared after the SBF immersion (Fig. 8),
The wide-angle XRD pattern of MCS/CPC after immersion in SBF for 7 days is shown in Fig. 9. It was observed that hydroxyapatite phase was present on the scaffold surfaces. This result was consistent with the results of SEM and EDS analyses.
Cultivation of C2C12 cells on scaffolds
Figure 10 shows that the viability of C2C12 cells on pure CPC scaffolds increased over the whole culture period by MTT assay. During 7 days of culture, viability of cells on MCS/CPC scaffolds remained similar.
In Figure 11 is plotted the C2C12 cells morphology after being seeded on MCS/CPC scaffolds for 3 days. The 3D visualization of the images revealed that the cells spread well with an intimate contact on the scaffold surface and almost covered the whole surface of the MCS/CPC strands.
Observation of cytoskeleton stained with FITC-Phalloidin (green) and nuclei stained with DAPI (blue) of C2C12 cells after 3 days of culture on the MCS/CPC scaffold. White columns inside the scaffold were caused by the light that penetrated the macropores from confocal laser scanning microscope (Color figure online)
Discussion
Fabrication of multifunctional scaffolds that meet structural, mechanical, and nutritional requirements is vital to direct 3D tissue ingrowth for the bone defect repair. In this study, MCS/CPC scaffolds with high mechanical strength were successfully fabricated by FFS.
Although FFS enabled artificial control of microstructures and affected mechanical properties, successful fabrication of CPC and MCS/CPC scaffolds highly depended on particles size, particle shape, binders’ viscosity, and the ratio of powder to binder of the paste. The fluidity and extrusion performance of paste were also crucial to fabricate 3D scaffolds. CPC and MCS powders were both preprocessed in advance to insure that the particle size was much smaller than the inner diameter of the printing nozzle. Being degradable, biocompatible, and water soluble, PVA (10 wt%) was used as the binder, which endowed the paste good fluidity. The setting reaction of CPC is a bidirectional process of dissolution and recrystallization of calcium phosphates. Once CPC-based powders mixed with aqueous solution, the setting reaction started. The fluidity of the CPC-based pastes would alter with time because of the formation of new setting products [34]. Pure CPC paste set so quickly that the nozzle of the printing system was easy to be blocked, which made the printable time of CPC paste short. Our previous study showed that the addition of CS with low crystallinity could markedly delay the setting time of composite cement because of its outstanding absorption [31]. It was also observed that the setting time obviously increased with the increase of the content of CS. In this study, MCS with large surface area and ordered mesoporous structure is highly absorbent. Furthermore, the addition of MCS would increase the distance between the TECP and DCPA grains, which meant increasing the distance for ions to diffuse and reducing the setting reaction rate. As a result, MCS/CPC paste with proper content of MCS gained enough time to print scaffolds with designed structure and showed excellent printability.
Besides chemistry, pore structure and mechanical strength are vital parameters for scaffolds. Although porous scaffolds with random pore size and shape distribution can be made easily with conventional techniques such as electro-spinning and foaming [35–37], pore interconnectivity and pore size of scaffolds cannot be controlled accurately. Even in the same batch, scaffolds possessed different pore structures. In addition, the fabrication processes spent too much time. Therefore, MCS/CPC scaffolds with uniformly interconnected pore were successfully fabricated by FFS. It has been reported that pore size of 150–500 μm is beneficial to facilitate cell infiltration, bone growth, and internally mineralized bone formation [38, 39]. Considering the balance between the tissue ingrowth and mechanical strength, MCS/CPC scaffolds with predefined pore size of 350 μm in X–Y direction were selected for further study.
The mechanical properties of bone tissue scaffolds are important because they are directly related to the structural constancy. A good mechanical strength should not only withstand external stresses during tissue generation, but also match the growth of tissues at the site of implantation. In general, macroporous geometry of scaffolds increases the amount of new bone but reduces the mechanical properties dramatically. The compressive strength of macroporous calcium silicate scaffolds fabricated by 3D extrusion-based printing technique was only 3.57 MPa [40], and the compressive strength of Ca-deficient hydroxyapatite porous scaffolds fabricated by particle-leaching method is about 1.79–6.46 Mpa [41]. However, the scaffolds fabricated here possessed a mechanical strength of 17.0 MPa, which was much higher than the porous scaffolds mentioned above. The reasons can be speculated as follows: (1) The high viscosity of PVA binder. PVA joined adjacent powder particles of the same and of neighboring layers, which made MCS/CPC scaffolds maintain the structure and do not collapse. (2) The self-setting characteristics of CPC. Once the MCS/CPC powder was mixed with PVA aqueous solution, the setting reaction began. After hardening at 37 °C in a 100 % humidity chamber for 72 h, the mechanical strength of MCS/CPC scaffolds was greatly improved (Fig. 5). (3) A more uniform and continuous pore structure produced by FFS. Generally, a uniform and continuous pore structure improves the mechanical strength [42].
Degradation is one of the most important characteristics for the scaffolds to be used in tissue engineering. As is shown in Fig. 6, the MCS/CPC scaffolds had higher weight loss than CPC scaffolds during the entire incubation period. On one hand, the faster degradation rate of MCS/CPC was mainly attributed to the quick dissolution of MCS due to its highly ordered mesoporous structure and large surface area. On the other hand, the incorporation of Ca into the mesoporous silicon produced some defects in the atomic array, such as atomic vacancies and dislocations, thereby resulting in the enhanced dissolution.
The formation of bioactive apatite layers is the prerequisite for biomaterial to bond with bone [43–45]. Therefore, the bioactivity of MCS/CPC scaffolds was tested in SBF by monitoring the apatite formation on their surfaces. SEM observation, EDS, and XRD analyses confirmed the newly formed hydroxy apatite layer on the surfaces induced by the MCS/CPC scaffold. Si peak vanished in the EDS spectra after the immersion process. It might be the following reasons which leaded to this: First, after being immersed in SBF for 7 days, plate-like crystallized apatite particles formed large aggregates and covered the surface of the scaffold. Second, the content of Si in the whole scaffold was relatively low. With the binder cohering the powder tightly, the releasing rate of Si ion might be slower than the depositing rate of Ca, P ion.
Cell culture experiments are able to provide cytocompatibility information of scaffolds for bone tissue engineering and regeneration. The viability and attachment ability of C2C12 cells seeded on CPC and MCS/CPC scaffolds were investigated in this study. The viability of cells seeded on the MCS/CPC scaffolds remained similar during the whole culture time, which indicated no cytotoxicity of MCS/CPC scaffolds. As observed by confocal laser scanning microscope, cell morphology suggested that the cells kept good activity after attaching to the surface of MCS/CPC scaffolds. It was demonstrated that high degree of supersaturation of Ca ions in the surrounding culture medium could result in cytotoxicity due to high content Ca ions released from scaffolds [1, 46]. Wu et al. [32] found that cell proliferation on mesoporous bioactive glass scaffolds was much lower than on the culture plate. The ordered mesoporous structure of MCS afforded not only the good solubility, but also the swift dissociation of Ca ions. As cells remained highly viable after being seeded on the CPC scaffolds, good cytocompatibility of CPC paste was testified here. We speculated that it was the blend with CPC that regulated the release of Ca ions from MCS/CPC, thus resulting in the good cytocompatibility of MCS/CPC scaffolds.
In a word, the MCS/CPC scaffolds with pore size of 350 μm fabricated by FFS showed properly interconnected macropores, high mechanical strength, fast degradation rate, and good cytocompatibility. In the ongoing studies, osteogenic ability of MCS/CPC scaffolds in vitro and in vivo will be further investigated.
Conclusion
We have successfully fabricated bioactive mesoporous calcium silicate/calcium phosphate cement scaffolds by freeform fabrication system with micro-droplet jetting. The MCS/CPC paste showed good printability. The developed MCS/CPC scaffolds presented completely interconnected macropores and precisely controllable outer shapes. MCS/CPC scaffolds with pore size of 350 μm showed high mechanical strength, fast degradation rate, and good cytocompatibility. These results indicated that the hybrid MCS/CPC scaffolds might be a promising candidate for critical bone defects.
References
Luo Y, Lode A, Sonntag F, Nies B, Gelinsky M (2013) Well-ordered biphasic calcium phosphate-alginate scaffolds fabricated by multi-channel 3D plotting under mild conditions. J Mater Chem B1(33):4088–4098
Lee G-S, Park J-H, Shin US, Kim H-W (2011) Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering. Acta Biomater 7(8):3178–3186
Bohner M (2007) Reactivity of calcium phosphate cements. J Mater Chem 17(38):3980–3986
Liu C, Gai W, Pan S, Liu Z (2003) The exothermal behavior in the hydration process of calcium phosphate cement. Biomaterials 24(18):2995–3003
Liu C, Shen W, Gu Y (1997) Mechanism of hardening process for a hydroxyapatite cement. J Biomed Mater Res 35(1):75–80
Liu C, Huang Y, Zheng H (1999) Study on the hydration process of calcium phosphate cement by impedance spectroscopy. J Am Ceram Soc 82(4):1052–1057
Liu C, Shen W, Chen J (2009) Solution property of calcium phosphate cement hardening body. Mater Chem Phys 58(1):78–82
Takagi S, Chow LC (2001) Formation of macropores in calcium phosphate cement implants. J Mater Sci 12(2):135–139. doi:10.1023/A:1008917910468
Seol Y-J, Park DY, Park JY, Kim SW, Park SJ, Cho D-W (2013) A new method of fabricating robust freeform 3D ceramic scaffolds for bone tissue regeneration. Biotechnol Bioeng 110(5):1444–1455
Leong KF, Cheah CM, Chua CK (2003) Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 24(13):2363–2378
Barralet JE, Grover L, Gaunt T, Wright AJ, Gibson IR (2002) Preparation of macroporous calcium phosphate cement tissue engineering scaffold. Biomaterials 23(15):3063–3072
Deville S (2008) Freeze-casting of porous ceramics: a review of current achievements and issues. Adv Eng Mater 10(3):155–169
Si Hesarak, Zamanian A, Moztarzadeh F (2008) The influence of the acidic component of the gas-foaming porogen used in preparing an injectable porous calcium phosphate cement on its properties: acetic acid versus citric acid. J Biomed Mater Res B 86B(1):208–216
Miao X, Hu Y, Liu J, Wong AP (2004) Porous calcium phosphate ceramics prepared by coating polyurethane foams with calcium phosphate cements. Mater Lett 58(3–4):397–402
Yeong W-Y, Chua C-K, Leong K-F, Chandrasekaran M (2004) Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol 22(12):643–652
Landers R, Mülhaupt R (2000) Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromol Mater Eng 282(1):17–21
Detsch R, Uhl F, Deisinger U, Ziegler G (2008) 3D-cultivation of bone marrow stromal cells on hydroxyapatite scaffolds fabricated by dispense-plotting and negative mould technique. J Mater Sci 19(4):1491–1496. doi:10.1007/s10856-007-3297-x
Miranda P, Pajares A, Saiz E, Tomsia AP, Guiberteau F (2008) Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. J Biomed Mater Res Part A 85A(1):218–227
Wu C, Luo Y, Cuniberti G, Xiao Y, Gelinsky M (2011) Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta Biomater 7(6):2644–2650
Fu Q, Saiz E, Tomsia AP (2011) Bioinspired Strong and Highly Porous Glass Scaffolds. Adv Funct Mater 21(6):1058–1063
Seyednejad H, Gawlitta D, Dhert WJA, van Nostrum CF, Vermonden T, Hennink WE (2011) Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater 7(5):1999–2006
Park S, Kim G, Jeon Y, Koh Y, Kim W (2009) 3D polycaprolactone scaffolds with controlled pore structure using a rapid prototyping system. J Mater Sci 20(1):229–234. doi:10.1007/s10856-008-3573-4
Lode A, Meissner K, Luo Y, Sonntag F, Glorius S, Nies B, Vater C, Despang F, Hanke T, Gelinsky M (2014) Fabrication of porous scaffolds by three-dimensional plotting of a pasty calcium phosphate bone cement under mild conditions. J Tissue Eng Regen Med 8(9):682–693
Klammert U, Reuther T, Jahn C, Kraski B, Kubler AC, Gbureck U (2009) Cytocompatibility of brushite and monetite cell culture scaffolds made by three-dimensional powder printing. Acta Biomater 5(2):727–734
Gbureck U, Hözel T, Klammert U, Würzler K, Müller FA, Barralet JE (2007) Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv Funct Mater 17(3):3940–3945
Habibovic P, Gbureck U, Doillon CJ, Bassett DC, van Blitterswijk CA, Barralet JE (2008) Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 29(7):944–953
Pati F, Jang J, Ha D-H, Kim SW, Rhie J-W, Shim J-H, Kim D-H, Cho D-W (2014) Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5:3935
Martínez-Vázquez FJ, Cabañas MV, Paris JL, Lozano D, Vallet-Regí M (2015) Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater 15:200–209
Zhu M, Li K, Zhu Y, Zhang J, Ye X (2015) 3D-printed hierarchical scaffold for localized isoniazid/rifampin drug delivery and osteoarticular tuberculosis therapy. Acta Biomater 16:145–155
Zhao Q, Qian J, Zhou H, Yuan Y, Liu C (2010) In vitro osteoblast-like and endothelial cells’ response to calcium silicate/calcium phosphate cement. Biomed Mater 5(3):035004
Guo H, Wei J, Yuan Y, Liu C (2007) Development of calcium silicate/calcium phosphate cement for bone regeneration. Biomed Mater 2(3):153–159
Wu C, Fan W, Gelinsky M, Xiao Y, Simon P, Schulze R (2011) Bioactive SrO–SiO2 glass with well-ordered mesopores: characterization, physiochemistry and biological properties. Acta Biomater 7(4):1797–1806
Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27(15):2907–2915
Liu C, Shao H, Chen F, Zheng H (2003) Effects of the granularity of raw materials on the hydration and hardening process of calcium phosphate cement. Biomaterials 24(23):4103–4113
Yokoyama Y, Hattori S, Yoshikawa C, Yasuda Y, Koyama H, Takato T, Kobayashi H (2009) Novel wet electrospinning system for fabrication of spongiform nanofiber 3-dimensional fabric. Mater Lett 63(9–10):754–756
Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK (2002) Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res 60(4):613–621
Dehghani F, Annabi N (2011) Engineering porous scaffolds using gas-based techniques. Curr Opin Biotechnol 22(5):661–666
del Real RP, Ooms E, Wolke JGC, Vallet-Regí M, Jansen JA (2003) In vivo bone response to porous calcium phosphate cement. J Biomed Mater Res Part A 65A(1):30–36
Xu HHK, Simon CG (2004) Self-hardening calcium phosphate cement–mesh composite: reinforcement, macropores, and cell response. J Biomed Mater Res Part A 69A(2):267–278
Wu C, Fan W, Zhou Y, Luo Y, Gelinsky M, Chang J, Xiao Y (2012) 3D-printing of highly uniform CaSiO3 ceramic scaffolds: preparation, characterization and in vivo osteogenesis. J Mater Chem 22(24):12288–12295
Guo H, Su J, Wei J, Kong H, Liu C (2009) Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater 5(1):268–278
Wu C, Ramaswamy Y, Boughton P, Zreiqat H (2008) Improvement of mechanical and biological properties of porous CaSiO3 scaffolds by poly(d, l-lactic acid) modification. Acta Biomater 4(2):343–353
Wu C, Chang J, Zhai W, Ni S (2007) A novel bioactive porous bredigite (Ca7MgSi4O16) scaffold with biomimetic apatite layer for bone tissue engineering. J Mater Sci 18(5):857–864. doi:10.1007/s10856-006-0083-0
Xu S, Lin K, Wang Z, Chang J, Wang L, Lu J, Ning C (2008) Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 29(17):2588–2596
Chou Y-F, Huang W, Dunn JCY, Miller TA, Wu BM (2005) The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials 26(3):285–295
Luo Y, Wu C, Lode A, Gelinsky M (2013) Hierarchical mesoporous bioactive glass/alginate composite scaffolds fabricated by three-dimensional plotting for bone tissue engineering. Biofabrication 5(1):015005
Acknowledgements
This investigation was supported by the National Basic Research Program of China (973 Program: 2012CB933600), National Natural Science Foundation of China (No. 31370960, No. 31100678), National Science & Technology Pillar Program during the Twelfth Five-years Plan Period (No. 2012BAD32B01).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, C., Gao, L., Chen, F. et al. Fabrication of mesoporous calcium silicate/calcium phosphate cement scaffolds with high mechanical strength by freeform fabrication system with micro-droplet jetting. J Mater Sci 50, 7182–7191 (2015). https://doi.org/10.1007/s10853-015-9244-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9244-1