Abstract
This paper discusses the synthesis and characterization of a new form of WO3 nanostructures based on the hexagonal WO3 (h-WO3) polymorph. This typically “metastable” phase was synthesized from a substoichiometric metal alkoxide precursor-tungsten (V) isopropoxide, by a novel colloidal synthesis method. Nanowires of h-WO3 were obtained upon heat-treating the sol–gel precursor at 400 °C. Further processing at elevated temperatures (515 °C) resulted in coarsening of the nanowires and the formation of nanowire bundles with polytypic character. Hexagonal WO3 differs from the other tungsten oxide polymorphs in that it possesses unique structural features such as long, hexagonal prism channels parallel to the c-axis and layered oxygen octahedra. This makes it a very attractive host matrix for metal ion intercalation for rechargeable batteries and for the selective gas sensing of amines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tungsten trioxide is a well-known metal oxide that finds widespread use in gas sensing, eletrochromic, and catalytic applications [1–3]. WO3 has a very interesting set of electronic properties where it can range from being a metallic conductor (in its highly reduced state) to an insulator. Polymorphic transformations of the pseudo-cubic lattice result in this wide variation in its electronic properties. WO3 usually crystallizes in one of the following crystal structure modifications- triclinic, monoclinic, orthorhombic, and tetragonal [4, 5]. All of these polymorphs are distorted forms of a cubic ReO3 lattice, with increasing order of crystallographic symmetry from the triclinic to tetragonal lattice. The crystal lattice is composed of a framework of metal–oxygen octahedra as depicted in Fig. 1, where the metal atoms are located at the center of the oxygen octahedra with varying amounts of metal–oxygen bond lengths and thus varying amounts of octahedral distortion [4, 5]. This distortion in turn serves to stabilize the different polymorphs.
Cubic WO3 with the ideal ReO3 lattice is difficult to obtain as a stable polymorph. The other thermodynamically metastable form of WO3 that has been synthesized and reported [5] has a hexagonal structure. The crystal structure is unique in that the lattice is made up of rings of corner sharing oxygen octahedra as depicted in Fig. 2. The layered oxygen octahedra provide both triangular and hexagonal prism channels that allow for easy movement of ions or gas molecules to travel through the lattice. Tungsten bronzes frequently crystallize in a hexagonal lattice, when the hexagonal tunnels are interpolated with specific cations [6]. But recently there have been reports of synthesis of pure h-WO3 obtained by dehydration of an orthorhombic WO3.1/3 H2O precursor. Since then, there has been a lot of interest in synthesizing h-WO3 as a matrix for intercalating metal ions for rechargeable batteries (Li+ [7]) and electrodes. Lithium ion batteries are vital for advancing the field of portable electronics. They operate by reversibly inserting Li+ ions from the electrolyte into the electrodes and in the process generating electricity. Reversible intercalation of Li+ ions into the host matrix is crucial for battery operation and can be accomplished by having electrode materials that have relatively open crystal structures [8]. Thermodynamically stable crystal structures are typically close-packed, whereas metastable oxide phases have open lattices that promote very high diffusion rates for intercalating ions.
In the area of resistive gas sensing, hexagonal WO3 is an attractive material due to the the structural similarity it shares with the orthorhombic form of MoO3 [8, 9] Both of these crystal structures have layered oxygen octahedra, i.e. an open lattice structure, that provides long paths for small, diffusing gas molecules and facilitates easy removal of oxygen ions from the lattice. The authors’ earlier research has established that the crystal structure plays a key role in determining the selectivity of the sensing matrix [8, 9]. Orthorhombic MoO3 has been shown to be selective to ammonia in the presence of other gases. MoO3 with its low sublimation temperature is not a suitable candidate for prolonged use at elevated temperatures. WO3 on the other hand has higher structural integrity than MoO3 and hence ideal for high temperature sensor applications. Also the high aspect ratio of the nanowires will serve to improve the energy density of the batteries without increasing the effective volume of the battery.
Furthermore, metal oxides with lower dimensionalities have been the focus of intense research activity for applications requiring high surface-area to volume ratio such as gas sensing, catalysis [10–12]. Nanotubes, nanowires, nanobelts and other one-dimensional nanostructures have most of their atoms as surface atoms, and hence provide more reaction sites for surface reactions. In particular, in the field of gas sensing, the absence of a bulk can tremendously increase sensor response and recovery times.
A review of the synthetic methods for obtaining hexagonal tungsten oxide was published recently [13]. In the past few years there have been a lot of research efforts to synthesize h-WO3 through different routes- vapor deposition [13], thermal evaporation [14], soft chemistry [15–17] to mention a few. In this work a novel and simple synthesis method for nanostructured hexagonal WO3 is described, involving the hydrolysis and subsequent annealing of a sub-stoichiometric metal alkoxide precursor in air. The influence of the annealing temperature on the film morphology has also been discussed.
Experimental methods
Tungsten (V) isopropoxide in isopropanol was the precursor used in this study. (The precursor was provided to the authors by Dr. H. Haneda and Dr. N. Ohashi of the Sensor Materials Center, at the National Institute of Materials Science (NIMS) at Tsukuba, Japan. The facilities used for the synthesis of the material were also housed at NIMS, where one of the authors (KK) carried out an internship).
The sol–gel precursor was drop-coated on glass substrates inside a glove box. The glass slides were ultrasonically cleaned in acetone for half-hour prior to film deposition. The as-deposited films inside the glove box were translucent yellow in color and upon contact with atmospheric moisture turned to a deep blue color. The films, once taken out of the glove box were immediately transferred to a furnace. The films were heat treated in air at 400–515 °C for various times. All the films were gray–white in color after the annealing treatment.
X-Ray Diffraction (XRD) on the thin films was carried out using a Philips PW1729 X-ray diffractometer with a Cu Kα radiation (λ = 1.54184 Å). Scanning electron microscopy (SEM) on the heat- treated films was carried out using a LEO GEMINI 1550 with a Schottky Field Emission Gun. The films were sputter coated with gold for 20 s before SEM observation. Transmission electron microscopy (TEM) was performed using a Philips CM12 STEM with a LaB6 cathode at an accelerating voltage of 120 keV. The samples used for TEM were prepared by scraping the film off the substrates and subsequently suspending the films in ethanol. The solution was then ultrasonically agitated to achieve a uniform dispersion. The solution was then drop-coated on to the TEM grids.
Results and discussion
Upon annealing the films were transformed to the hexagonal WO3 phase after 6 h at 400 °C. Further treatment at higher tempreratures and longer times established the 515 °C for 8 h as the upper limit for the presence of the hexagonal phase. At this stage the nanostructures appear to coarsen significantly.
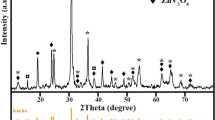
Figure 3 corresponds to the XRD pattern of the film heat treated at 400 and 515 °C, respectively. The film heat treated at 400 °C gave XRD peaks can be indexed to match the hexagonal WO3 corresponding to the JCPDS card number 85-2459 with unit cell dimensions of a = 7.3244, c = 7.6628. This phase is similar to the one reported by Solonin et al. [18]. Neither tungsten oxide hydrate nor hydrogen tungsten oxide peaks were observed in the XRD patterns. More peaks of the same structure may be resolved in the spectrum of the specimen heat-treated at 515 °C.
The corresponding scanning electron images for the two sets of samples for which the XRD data are given above are seen in Fig. 4 for the 400 °C heat-treated sample, and Fig. 5 for the 515 °C one. Both films exhibit cracking, typical of sol–gel processed thin films following an annealing treatment.
Figure 5 Scanning electron micrographs showing the bimodal distribution of the hexagonal structures in the sample heat-treated at 515 °C for 8 h. The nanowires have either distinct presence or are seen in bundles of shorter length and flat edges.
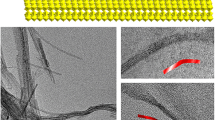
The film heat-treated at 400 °C consists of nanowires and some aggregates. The film heat-treated at 515 °C on the glass substrate manifests coarsening of the nanowire structures. Distinct, single crystal nanowires are also seen that have grown to 40 nm in width/diameter and up to 2–5 μm in length (see Fig. 6a). As can be observed from the selected area diffraction (SAD) pattern (Fig. 6a inset), the main spots can be indexed to the (001) family of planes of the h-WO3 crystal structure, thus indicating that the nanowires grow along the (001) direction. Also the absence of extra spots in the SAD pattern indicates that the nanowires are single crystals.the TEM.
What appears in Fig. 5c as nanoparticles covering 40 % of the film area, are actually nanowires aggregated into bundles as seen and described by other researchers for hexagonal tungsten trioxide nano-morphologies [19]. Such a nanowire bundle is imaged in Fig. 6b in the TEM to have a hexagonal morphology and exhibit clear facets. The inset shows the corresponding SAD pattern.. The presence of extra spots in the diffraction pattern suggests polytypic growth. The presence of shear planes is not restricted only to the pseudo-cubic crystal structures of WO3, but they can also be accommodated in the hexagonal lattice without resulting in loss of stoichiometry [16].
Proposed synthesis method for the hexagonal-WO3 nanostructures
Based on the principles of sol–gel processing, the reaction expected to occur when tungsten (V) isopropoxide (W( iPr) 5) comes in contact with moisture is given below:
The hydrolysis and subsequent condensation in this case occurs by removal of water. The isopropoxide functional group is removed as isopropanol, which then dries out. The blue color of the precursor on hydrolysis may be explained by the electron excess on the W 5+ ion and the electron excess can be accommodated by oxygen vacancies. Though detailed spectroscopic studies on the intermediate precursor are needed, a viable mechanism for the formation of hexagonal tungsten trioxide is proposed here. The precursor for the hexagonal lattice requires an additive for stabilizing the framework. Previous reports suggest that either a hydrate or hydrogen substituted metal oxide is essential in the formation of hexagonal tungsten oxide to serve as the intermediate step. The water molecule in the hydrate or the hydrogen atom in the case of the metal hydrogen oxide serves to stabilize the hexagonal lattice, which is otherwise thermodynamically unstable. The substoichiometric isopropoxide precursor on reaction with the moisture in the atmosphere likely results in the formation of H0.24WO3, which has been reported as the intermediate product for the synthesis of h-WO3 in studies by other workers [18], who synthesized the same phase of hexagonal WO3 on oxidation in air. A hydrogen substituted metal oxide is more viable as a precursor in this case as compared to the hydrate. This is because of the fact that the metal alkoxide is sub-stoichiometric and removal of two molecules of water from the W(OH)5 in Eq. (1) results in a lone hydrogen atom that can be easily accommodated in the hexagonal framework of WO3 as shown in Fig. 7a which then transforms to h-WO3 (Fig. 7b).
Earlier work on self assembled h-WO3 nanowire bundles suggests that the presence of a catalyst ion such as a sulfate, on the faces parallel to the c-axis leads to preferential growth along the c-axis [19]. This seed crystal serves as the precursor for the growth of long one-dimensional structures. However, in this case there is no catalyst ion addition added to the precursor.
Polymorphic reactions may drive nanowire growth in the absence of a catalyst, as it was recently shown in the in situ growth in the TEM of monoclinic WO3 nanowires from a cubic WO3 sol–gel processed precursor phase [20]. Similarly, in our earlier work on molybdenum oxide we observed orthorhombic MoO3 growth from sol–gel precursors as long, single-crystal nanowires [12]. The colloidal precursor solution apparently supplied the building blocks for the nanowire growth.
In conclusion, we report the synthesis of nano h-WO3 without any catalyst or impurity additions by a simple annealing treatment from a metal alkoxide precursor. The formation of this thermodynamically metastable polymorphic modification is likely to have involved a hydrogen-substituted intermediate crystal lattice, that eventually transformed to h-WO3. The nanowires structures and their aggregates provide a high surface area to bulk volume ratio and hence are readily amenable for applications that require high surface areas such as gas sensing, catalysis and power applications. The presence of unique crystallographic features such as the long prism tunnels and unique layered morphology in h-WO3 is expected to provide the new level of selectivity for discriminative gas sensing and also serve as an intercalation host for rechargeable Li+ batteries.
References
Lietti L, Alemany JL, Forzatti P, Busca G, Ramis G, Giamello E, Bregani F (1996) Reactivity of V2O5-WO3/TiO2 catalysts in the selective catalytic reduction of nitric oxide by ammonia. Catal Today 29:143–148
Livage J, Guzman G (1996) Aqueous precursors for electrochromic tungsten oxide hydrates. Solid State Ion 84:205–211
Gouma P (2011) Nanoscale Polymorphic Oxides for Selective Chemosensors. Sci Adv Mater 3(5):787–793
Wang L, Gouma P (2013) Selective microstructure synthesis and sensing dependencies: a WO3 study. In: Carpenter MA, Mathur S, Kolmakov A (eds) Metal oxide nanomaterials for chemical sensors. Springer, New York
Gerand B, Nowogrocki G, Guenot J, Figlarz M (1979) Structural study of a new hexagonal form of tungsten oxide. J Solid State Chem 29:429–434
Tilley RJD (1995) The crystal chemistry of the higher tungsten oxides. Int J of Refract Met Hard Mater 13:93–109
Slade RCT, West BC, Hall GP (1989) Chemical and electrochemical mixed alkali metal insertion chemistry of the hexagonal tungsten trioxide framework. Solid State Ion 32–33:154–161
Whittingham MS, Guo JD, Chen R, Chirayil T, Janauer G, Zavalij P (1995) Hydrothermal synthesis of new oxide materials. Solid State Ion 75:257–268
Gouma PI, Prasad AK, Iyer KK (2006) Selective nanoprobes for ‘signaling gases’. Nanotechnology 17:S48–S53
Comini E, Guidi V, Malagù C, Martinelli G, Pan Z, Sberveglieri G, Wang ZL (2004) Electrical properties of two dimensional tin oxide nanostructres. J Phys Chem B 108:1882–1887
Gouma PI, Kalyanasundaram K, Bishop A (2006) Electrospun single-crystal MoO3 nanowires for biochemistry sensing probes. J Mater Res 21:2904–2910
Han W, Hibino M, Kudo T (1998) Synthesis of the hexagonal form of tungsten trioxide from peroxopolytungstate via ammonium paratungstate decahyrate. Bull Chem Soc Jpn 71:933–937
Gillet M, Delamare R, Gillet E (2005) Growth of epitaxial tungsten oxide nanorods. J Cryst Growth 279:93–99
Wu Y, Xi Z, Zhang G, Yu J, Guo D (2006) Growth of hexagonal tungsten trioxide tubes. J Cryst Growth 292:143–148
Gu Z, Li H, Zhai T, Yang W, Xia Y, Ma Y, Yao J (2007) Large-scale synthesis of single-crystal hexagonal tungsten trioxide nanowires and electrochemical lithium intercalation into the nanocrystals. J Solid State Chem 180:98–105
Choi HG, Jung YH, Kim DK (2005) Solvothermal synthesis of tungsten oxide nanorod/nanowire/nanosheet. J Am Ceram Soc 88:1684–1686
Balászi Cs, Prasad AK, Pfeifer J, Tóth AL, Gouma PI (2005) Wet Chemical synthesis of nanosize tungsten oxide for sensing applications. In: Pődör B, Horváth Zs, Basa P (eds) Proceedings of the first international workshop on semiconductor nanocrystals SEMINANO2005, Budapest, Hungary, pp 79-82
Solonin YM, Khyzhun OY, Graivoronskaya EA (2001) Nonstoichiometric tungsten oxide based on hexagonal WO3. Cryst Growth Des 1:473–477
Gu Z, Ma Y, Yang W, Zhang G, Yao J (2005) Self-assembly of highly oriented one-dimensional h-WO3 nanostructures. Chem Commun 28:3597–3599
Sood S, Kisslinger K, Gouma P (2014) Nanowire Growth by an Electron-Beam-Induced Massive Phase Transformation. J Am Ceram Soc 97(12):3733–3736
Acknowledgements
The work was partially supported through a NIMS Internship Program award to K.K., sponsored by the National Institute of Materials Science, Tsukuba, Japan and by the NSF award DMR1106168 to P. Gouma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gouma, P.I., Kalyanasundaram, K. Novel synthesis of hexagonal WO3 nanostructures. J Mater Sci 50, 3517–3522 (2015). https://doi.org/10.1007/s10853-015-8918-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8918-z