Abstract
Nanoblending hydrophilic nanofillers thoroughly into hydrophobic polymer matrices has long been challenging, especially if involving no pre-functionalization on a 1D ceramic nanomaterial. Here we report a facile approach to fine-tuning of sodium titanate (Na2Ti3O7) nanobelt’s (NB) surface chemistry widely by exchanging the NB’s crystal lattice cations, for successfully nanoblending the low-cost and versatile NBs into the poly(vinyl benzyl chloride) or p(VBC) and the sulfonated form of pVBC’s [or sp(VBC)] matrixes. For the first time, the adjustable nanocompositing showed a long-sought workability in not only in situ radical polymerization of VBC monomer but also ex situ nanoblending of the p(VBC), with the NBs. The resultant nanocomposites possess an unusual surface versatility that can be tailored from being hydrophilic to being hydrophobic by design. This method concludes a generalized and industry-viable approach to mass-producing nanocomposites of many types facilely at low-cost, especially for large scale industries such as packaging materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer-based nanocomposites have received much attention lately [1–3], thanks to their unusual potentials in important applications such as water-treatment [4], anticorrosion [5], supercapacitors [6], microwave absorption [7–9], to name but a few. In this burgeoning field, nanoscale fillers have been proven crucial in significantly improving the nanocomposites’ structure and surface properties such as mechanical strength [10], thermal stability [11, 12], and electrical conductivity [13]. Among the nanofillers, one-dimensional (1D) nanomaterials (e.g., nanobelts, nanowires, etc.) have attracted great interest, because they hold a great promise in improving the polymer crystallinity by spatially aligning the otherwise entangled polymer-chains in sub-nm scale. Intuitively, a mild increase in crystallinity can dramatically improve a polymeric material’s physical and chemical properties, e.g., gas-permeability, glass-transition temperature, Young’s modules, etc., turning a low-cost polymer resin into a large suite of multipurpose and value-added new materials.
In order to homogeneously disperse a nanofiller in a polymeric matrix, one has to tune the nanofiller’s interfacial chemistry comparable to that of the polymeric matrix, based on the simple chemical principle of “like ‘dissolves’ like,” which is intrinsically challenging to many nanofillers.[14] In contrast to enormous efforts in using near-spherical or irregularly shaped nanofillers that are relatively easy to access, nanoblending low-cost biocompatible one-dimensional (1D) nanofiber in polymeric matrix has been relatively underexploited. This is in part because the 1D nanofiller may readily self-entangle to aggregate forms especially in the polymer matrix, [15, 16] resulting in a severe phase-separation and the nanoscale inhomogeneity.
Titanate nanobelt (NB) is an inexpensive and non-toxic 1D nanomaterial that can provide us with a variety of highly translational properties (e.g., high reflective index [17], thermal stability [18], wide band gap [19], etc.). In terms of interfacial nanoscience, this NB is in fact surfaced by alternating positive-charged layers of exchangeable Na+ cation and negative-charged titanate polyoxoanionic layers, which is different than most other types of 1D nanomaterials. As a result of the unusual lattice structure, the sodium titanate NB shows a good hydrophilicity, which prompted us to wonder if we could fine-tune the hydrophilicity–hydrophobicity via controlling the interlayer cation type. This inspiration motivated us to explore how to optimally nanoblend a hydrophilic inorganic filler into a hydrophobic polymer matrix, since this is a longstanding challenge in maximizing an ordinary polymer’s structural and interfacial as well as functional versatilities at low-cost and precisely as designed.
In the literature, progresses have been ongoing in simply mixing TiO2-based fillers in polymer matrix [20, 21], with a few works involving the titanate-based 1D nanomaterials [22–24]. On the other hand, core–shell structured polymer nanocomposites have been successfully synthesized via grafting poly(methyl methacrylate) (PMMA) in silica through a surface-initiated polymerization [25]. The greatly improved overall structural stability of these polymer nanocomposites mostly resulted from the well-tuned interfacial chemical bonding between the polymeric matrix and the fillers.
In compositing hydrophilic 1D nanofillers with hydrophobic polymers, which is probably among the most challenging in nanocompositing, a common practice is to pre-functionalize the nanofiller surface with expensive chain-like molecules (e.g., organosilanes) with hydrophobic end-groups that can better interface with the hydrophobic polymer matrix. Thus, omitting the surface-functionalization step is economically advantageous and technologically difficult, hence seldom reported in the literature. This work was based on such hypotheses that the uniquely tunable surface and the 1D morphology of the titanate NB can result in (i) unique chemical bonding and/or nanoblending with the polymer matrix containing active groups such as chlorine atom present vinyl benzyl chloride or sulfonic group in the sulfonated polymer, and hence (ii) an unusual spatial organization of the polymer backbone which enhances the hydrophobic character of the TiO2 nanobelts. The aim of this study was to modify the surface of the titanate NBs using either poly vinyl benzyl chloride or its sulfonated form, and then to evaluate the nanoscale modification’s effect on the nanofiller’s nanoblending inside the polymer matrix. Among all possible ways of forming the nanocomposite, such a nanografting strategy developed in this work was proven to be the most facile and economic [26].
Experimental section
Materials
4-Vinyl benzyl chloride (VBC) monomer was obtained from Acros Organics (Tech. grade, 90 %) and purified using sodium hydroxide to remove the free-radical inhibitor. TiO2 (P25) was supplied by Degussa. Benzoyl Peroxide (98 %,) was a product of Alpha Chemica. Calcium Carbonate (98.5 %) was purchased from OXFORD laboratory. All solvents such as toluene (99.8 %, M-TEDIA), methanol (99 %, ADWIC), methylene chloride (99.5 %, TEDIA), and tetrahydrofuran (95 %, SDFCL-sd fine chem. Limited), were used as received.
Synthesis of polyvinyl benzyl chloride [p(VBC)]
The polymerization of VBC was carried out via a well-established protocol [27]. 0.2 g (8.2 × 10−4 mol) of benzoyl peroxide and 40 mL of toluene were added to a 250 mL 3-necked round-bottomed flask. Oxygen was removed by flushing with dry nitrogen for 5 min, and then 8 mL of 4-VBC was added by a syringe. The polymerization took place at 95 °C for 2 h. The resulting polymer was precipitated in 200 mL methanol after cooling. The precipitate was then purified by three cycles of the dissolution/precipitation. Finally, the resultant poly 4-vinylbenzyl chloride or p(VBC) was dried at 60 °C under vacuum.
Synthesis of sulfonated poly vinyl benzyl chloride sp(VBC)
The sulfonated polyvinyl benzyl chloride was prepared by following the method in literature [28]. 103 µL of 3.3 × 10−3 M H2SO4 was transferred to a 50 mL round-bottom flask, and then 0.02 g of P2O5 was slowly added in under stirring, then the mixture was cooled to 40 °C. Then, 1.00 g of p(VBC) was dissolved in 10 mL of CH2Cl2, then added drop wise to the H2SO4–P2O5 mixture to initiate the reaction. After 30 min, the reaction was stopped and allowed to sit still at 40 °C for 1 h. Thereafter, the bottle was cooled via adding ~2.0 g of crushed ice while stirring, resulting in the sulfonated polymer in the form of a pale-yellowish solid. The reaction mixture was then separated and washed with distilled water, and the solid polymer was finally collected and dried at 60 °C overnight under vacuum.
Synthesis of intercalated nanobelts (Na2Ti2O7-NBs)
The titanate NBs were prepared following our patented method [29]. 0.2 g of TiO2 nanoparticles (P25) was added to 40 mL (10 M) sodium hydroxide in a Teflon-lined autoclave, heated at 240 °C for 72 h to form the white precipitate which represents the raw NBs with the chemical composition of Na2-nHnTi3O7. The raw NBs were filtered, washed with 1.0 M NaCl until pH ~7.0, and stirred (5000 rpms) in 1.0 M NaCl for 72 h to form the Na2Ti3O7-NBs which is finally washed and dried overnight at 80 °C.
Ex situ synthesis of the nanocomposite of sp(VBC)/Na2Ti3O7-NBs
In 15 mL of THF, 0.2 g (8.5 × 10−4 mol) of the sulfonated p(VBC) or sp(VBC) was first dissolved, and then 0.24 g (8.5 × 10−4 mol) of Na2Ti3O7-NBs was added. The mixture was sonicated for 30 min, and then vigorously stirred for a week at the stirring rate of 1000 rpm. Through this week, samples were collected based on the stirring 2, 4, and 7 days, respectively. Each such sample was washed 3 times with THF using centrifuge, and dried at 50 °C overnight in vacuum.
In situ synthesis the p(VBC)/Na2Ti3O7-NBs nanocomposite
The p(VBC)/Na2Ti3O7-NBs nanocomposites were prepared via an in situ polymerization of VBC monomer in the presence of the NBs. Here, a 3-neck round-bottom flask was flushed and filled with N2 then charged with 1.7 mL of free-radical inhibiting 4-VBC, 0.5 g of the Na2Ti3O7-NBs, and 15 mL toluene. The whole mixture was sonicated for 30 min, and stirred (5000 rpm) for 72 h. Then, 0.04 g of benzoyl peroxide was added to the mixture, and the polymerization reaction was carried out at 95 °C for 1 h. The resulting nanocomposite was precipitated in 200 mL methanol, and then washed using methanol and distilled water for multiple times. Finally, the nanocomposite of pVBC/Na2Ti3O7-NBs was dried at 70 °C under vacuum.
Ex situ synthesis of the p(VBC)/Na2Ti3O7-NBs nanocomposite
In 15 mL of THF, 0.36 g (2.37 × 10−3 mol) of p(VBC) was dissolved, and then 0.5 g of Na2Ti3O7-NBs was added. Separately, 0.54 g (3.6 × 10−3 mol) of p(VBC) was dissolved in 15 mL of THF, then 1.0 g of Na2Ti3O7-NBs was added. After sonicating the two mixtures for 30 min, each went through a vigorous stirring (1000 rom) for 1 week. Through this week from each of the two mixtures, three samples were collected at the day intervals of 2, 4, and 7, respectively, and each sample was washed with THF for 3 times using centrifuge, then dried at 50 °C under vacuum overnight.
Characterizations
The structures of the prepared nanocomposites were examined using JEOL JEM-1230 transmission electron microscope (TEM) with acceleration voltage of about 80 kV. The surface morphology was examined using JEOL-HRSEM scanning electron microscope (SEM). The thermal behavior was studied using Simultaneous DSC-TGA instrument, or SDT Q600 under nitrogen atmosphere at a heating rate of 10 °C/min. XRD patterns were recorded by Diano X-ray diffractometer with Cu Kα-radiation source energized at 45 kV. 1H NMR spectra were acquired on JEOL-ECA 500 MHz spectrometer in DMSO at 25 °C with tetramethylsilane (TMS) as an internal standard. Fourier Transform Infrared Spectrometer (FTIR-6100) was used to investigate the chemical structure of the prepared polymers and nanocomposites. Molecular weight of the polymer was determined by gel permeation chromatography (GPC)-Agilant instrument, using polystyrene standard, refractive index (RI) detector, and flow rate of 1 mL/min and dimethyl acetamide (DMAc) as eluent. Dynamic contact angle measurements were carried out using Data Physics OCA35L/SCA202, measuring liquid is distilled water and the calculation method is ellipse fitting, and the samples were prepared by spin coating onto a glass substrate.
Results and discussion
Structure and morphology of the intercalated titanate NBs
The formation of white-colored 1D nanobelts, sodium titanate form (Na 2 Ti 3 O 7 )-NBs, based on our published work in this field [22], is strongly supported by the SEM and XRD data.
Fig. 1a depicts diffraction patterns of the typical layered titanate for the two NB-samples, one before and the other after the intercalation, with each showing the characteristic diffraction peaks at 2θ ≈ 10° (001), 2θ = 25° (110), and 2θ = 29.7° (003). In fact, the Na2Ti3O7 NB’s XRD peak at 2θ ≈ 10° is slightly shifted toward a lower angle (or a higher d-spacing), which is owing to the bigger size of Na+ compared to H+ in the layered lattice after the intercalation [30]. Fig. 1b shows an SEM micrograph of the smoothly surfaced NBs, and confirms that the NB’s crystal structure and morphology were kept intact even after the thorough Na+ intercalation. The inset in Fig. 1b is a TEM image that shows a thus-formed NB’s average diameter of 33 nm.
In situ formation p(VBC)/Na2Ti3O7-NBs nanocomposite
The chemical structure of pVBC was confirmed by FTIR = 1262 (cm−1) for C–Cl, 2921 (cm−1) for CH2 (stretching), 3019 (cm−1) for C–H (stretching) in phenyl groups. The investigated structural analysis with 1H NMR using DMSO-d6 as a solvent showed the following peaks: δ = 0.8 (CH2), δ = 4.58 (CH2Cl), δ = 6.5–7.2 (protons of Aromatic ring), and δ = 1.6 (CH).
Molecular weight determination with GPC revealed that the obtained polymer has M n = 7700 g/mol and polydispersity index (PDI) = 1.8.
In the FTIR spectra of the nanocomposites (Fig. 2a), the characteristic strong absorption band at 1262 cm−1 should be due to the bending vibration of C–Cl in the CH2–Cl group in the p(VBC) [2]. The aromatic C–C stretching can be observed at 1470 cm−1. The absorption band at 2920 cm−1 is from the anti-symmetric stretching vibration of –CH2– group of the polystyrene chains. The absorption band at 3020 cm−1 is corresponding to C–H stretching of aromatic rings. The Ti–OH bond is evidenced by the peak at 3400 cm−1, which confirms the existence of hydroxyl groups on the NB [2]. Moreover, bands at 850 and 500 cm−1 can be assigned to the Ti–O–Ti stretching vibrations. Chemically, the Cl atom in the monomer has the affinity to bind to the Na+ cations that are linearly aligned on the NB-surface, which in turn can help (i) disperse the NB inside the polymer matrix and (ii) line up the polymer backbones along the NB-longitudinal axis.
Accordingly, the in situ free-radical polymerization of VBC directly on the Na2Ti3O7-NBs was proven successful in the process of forming the p(VBC)/Na2Ti3O7-NBs nanocomposites, without the help of any surfactant for dispersing the NBs in the VBC, which is truly novel in the slow-developing field of using low-cost and versatile nanomaterials to improve ordinary polymers’ properties. Obviously, prior to the polymerization the three-day stirring in the VBC monomer solution led to the significant “wetting” of NBs inside the VBC in order to nanoblend the NBs well with the pVBC. Consequently, this method can be considered an effective route for fine-tuning a 1D nanomaterial’s surface as such for realizing the long-sought nanoblending with the hydrophobic polymer.
Morphologies of the nanocomposites from the raw NBs were found to be highly different than that from sodium titanate NBs. Based on the SEM micrograph (Fig. 2b), the polymer formed a relatively smooth coating on the Na-intercalated nanofibers. This is in line with the ionic interaction between chloride anions and sodium cations, as suggested before. The inset TEM image shows the homogenous surface coating and increase of NB diameter to 100 nm. On the raw NB’s surface, however, the mixed Na+ and H+ probably “confused” the polymer backbone, resulting in an agglomeration-like surface with short NBs (Fig. 2c), implying a nanoscale phase-separation or poor nanoblending on the NB may break the long NBs into short pieces. Nevertheless, the average diameter of the raw NB is increased to 123 nm as revealed by TEM (inset Fig. 2c). In addition, if under this experimental condition the polymer or its monomer could penetrate into the interlayer space of the NB’s layered lattice, the NB’s crystal lattice could be exfoliated. Nonetheless, the Na+ and H+ co-existing on the raw NB’s surface can interact with the polymer backbone very differently through electrostatic attraction forces with the electronegative Cl atom found in the polymer structure. This better adhesion and dispersion is new and useful in nanocompositing 1D nanofibers with polymers with no need for compatiblizers.
The NB’s possible exfoliation and length-shortening can be verified by means of XRD study. In the XRD patterns (Fig. 3a), the characteristic diffraction peaks of the NBs show intensities significantly reduced in the nanocomposites when compared with their non-composite forms. This result suggests that (i) the polymer-coating put onto the NB-surface with much strain that in turn reduced the thin NB’s crystallinity, hence (ii) the better and smoother coating on the Na+-intercalated NB-surface corresponds to the larger strain and in turn the worse crystallinity or the lower XRD-peaks’ intensity for the NBs. In fact, the broadening of the XRD peaks in the nanocomposites from the reduced crystallinity (Fig. 3a) is in line with shorter NBs (see SEM results in Fig. 2).
Furthermore, the XRD patterns (near two-theta of 10°) showed that the NBs in the nanocomposites possess a reduced d-space, due most likely to the loss of hydrated water around each interlayer cation during the formation of the nanocomposites. In other words, since no XRD peak at lower 2-theta occurs for the dried samples, the NB-lattice’s exfoliation was negligible.
Ex situ formation of p(VBC)/NB and sp(VBC)/NB nanocomposites
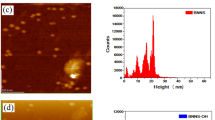
The nanocomposites based on p(VBC) and sp(VBC) were successfully prepared by an ex situ method for comparison. It was found that after 7 days the ordinary p(VBC)’s coverage on the Na titanate NBs (Fig. 3b,c; different) is far less complete than the sp(VBC)’s (Fig. 3d), which suggests that the sulfonic group did enhance the interaction between the NBs and the polymer backbone during the ex situ polymerization. In other words, it was the very sulfonated group that enabled us to better materialize the homogeneous nanoblending readily, as shown by the TEM micrographs (Fig. 3d) and SEM images (Fig. 4a,b).
These results prompted us to conduct a time-study. In the experiment, the Na-intercalated titanate NBs were sonicated with the sulfonated form of p(VBC) then stirred for 1 week, during which the nanoblending degree and formation of of sp(VBC)/Na2Ti3O7-NBs were checked with XRD at different time intervals during the 7 days. From the XRD patterns (Fig. 4c), one can find that (i) the main peak’s intensity decreases by increasing the reaction time, and (ii) the main peak’s position slightly shifted from 2θ of 9.86 to a 2θ reading 10.7. In parallel, the NB-size reduced from 11.08 nm at the time interval of 4-days to 10.23 nm at the 7-days, which implies a new way for us to expand our toolbox for tailor-making of a nanocomposite by fine-tuning the NB’s structural and in turn the exciting interfacial properties inside a polymer matrix.
TGA data for both the NBs and the nanocomposites (see Fig. 5a and Table 1) strongly support the results discussed in the above. The increased thermal stability of Na-intercalated titanate NBs, in comparison with the as-made (or raw) NBs, suggested that the reduced number of hydrate-water molecules around a larger cation at the interlayer space significantly stabilized the layered lattice. This trend is in line with the diameter-trends for dehydrated cations (i.e., Li+ < Na+ < K+) and hydrated cations (i.e., Li +(aq) > Na +(aq) > K +(aq) ),[31, 32] because (i) a smaller cation possesses a higher charge-density to attract more water molecules (i.e., bigger in diameter) and such water molecules can be removed at lower temperature.
Table 1 shows temperatures that caused 10 % weight loss for all samples. Since smaller proton could hold more water molecules, as discussed in the above, the first 10 % weight loss from the as-made NBs occurred at the temperature much lower than that from the Na-intercalated NBs (see Table 1).
The p(VBC) nanocomposite with the as-made NB shows two stages of weight loss. The first stage is attributed to the elimination of HCl which has onset at 185 °C, while the second stage is attributed to degradation of the polymer backbone near 330 °C, based on our previous experience and the literature. For the nanocomposite of sulfonate p(VBC) with the Na+-titanate NBs, however, three steps of the weight loss were recorded. The first step is a linear decrease in weight with increasing temperature due to elimination of water, the second one up to 440 °C due to elimination of small molecules such as HCl, and the third corresponds to polymer backbone’s degradation with the highest degradation temperature near 580 °C. Table 1 lists all these samples’ 10 % weight loss temperatures, which from another angle reflects the same trends of structural thermal stability mentioned in the above.
In addition, the dynamic contact angle was measured for checking the surface hydrophilicity/hydrophobicity for the NBs and the nanocomposites, based on the known chemistry that the NB-surface is hydrophilic, while the p(VBC)-surface is hydrophobic. According to Fig. 5b, Na-NB’s nanocomposite with p(VBC) is hydrophobic, with the contact angle reaching 99°, which supports that hydrophobic polystyrene chains in the p(VBC) arranged to cover the NB-surface. With the presence of hydrophilic phosphate group, the contact angle became 50°, which is closer to the highly hydrophilic NB’s contact angle of 25°. This set of data indeed suggests a range of tuning the resultant nanocomposite’s hydrophobicity/hydrophilicity in between 50° and 99° via adjusting the sulfonate group’s content in the p(VBC), which has been seldom demonstrated in making new nanocomposites.
Conclusion
Nanoblending a low-cost and hydrophilic 1D-nanofiber into a hydrophobic polymer matrix, a task long regarded highly challenging, was demonstrated doable in this work that involved no expensive equipment and harsh experimental condition, which can be generally applicable although seems counterintuitive. The thermal stability and surface hydrophobicity/hydrophilicity of the obtained nanocomposites were both found to be tunable widely through using of p(VBC) or its sulfonated form as modifiers. These results reveal the ability of controlling the structure and the function of nanobelt-based composite. Under the inspiration of this work, versatile nanocomposite membranes have been developed to show controllable solvent-crossover and charge-conduction, the long-sought properties crucial in making high-temperature proton-exchange membrane fuel cell (PEMFC). Moreover, these nanocomposites can find applications in mass production of polystyrene packing materials industry. By adding the newly surface modified hydrophobic TiO2 nanobelt to polystyrene matrix, better dispersion can be acquired and new properties of the final product can be achieved.
References
Bell CA, Smith SV, Whittaker MR, Whittaker AK, Gahan LR, Monteiro MJ (2006) Surface-functionalized polymer nanoparticles for selective sequestering of heavy metals. Adv Mat 18(6):582–586
Haroun A, Youssef AM (2011) ’Synthesis and electrical conductivity evaluation of novel hybrid poly (methyl methacrylate)/titanium dioxide nanowires. Synth Met 161(19–20):2063–2069
Xu G-C, Li A-Y, Zhang L-D, Wu G-S, Yuan X-Y, Xie T (2003) Synthesis and characterization of silica nanocomposite in situ photopolymerization. J Appl Polym Sci 90(3):837–840
Patel H, Somani R, Bajaj H, Jasra R (2006) Nanoclays for polymer nanocomposites, paints, inks, greases, and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull Mat Sci 29(2):133–145
Yeh J, Chang K (2008) Polymer/layered silicate nanocomposite anticorrosive coatings. J Ind Eng Chem 14(3):275–291
Frackowiak E, Khomenko V, Jurewicz K, Lota K, Beguin F (2006) Supercapacitors based on conducting polymers/nanotubes composites. J Power Sources 153(2):413–418
Mdarhri A, Brosseau C, Carmona F (2007) Microwave dielectric properties of carbon black filled polymers under uniaxial tension. J Appl Phys 101(8):084111
Mdarhri A, Carmona F, Brosseau C, Delhaes P (2008) Direct current electrical and microwave properties of polymer-multiwalled carbon nanotubes composites. J Appl Phys 103(5):054303
Qin F, Brosseau C (2012) A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles. J Appl Phys 111(6):061301
Fox J, Wie JJ, Greenland B-W, Burattini S, Hayes W, Colguhoun HM, Mackay ME, Rowan SJ (2012) High-strength, healable, supramolecular polymer nanocomposites. J Am Chem Soc 134(11):5362–5368
Ramanathan T, Abdala AA, Stankovich S, Dikin DA, Herrera-Alonso M, Piner RD, Adamson DH, Schniepp HC, Chen X, Ruoff RS, Nguyen ST, Aksay IA, Prud’Homme RK, Brinson LC (2008) Functionalized graphene sheets for polymer nanocomposites. Nat Nanotechnol 3(6):327–331
Brosseau C, Boulic F, Queffelec P, Bourbigot C, Le Mest Y, Loaec J, Beroual A (1997) Dielectric and microstructure properties of polymer carbon black composites. J Appl Phys 81(2):882
Du F, Scogna RC, Zhou W, Brand S, Fischer JE, Winey KI (2004) Nanotube networks in polymer nanocomposites: rheology and electrical conductivity. Macromolecules 37(24):9048–9055
Guo Z, Pereira T, Choi O, Wang Y, Hahn H (2006) Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties. J Mat Chem 16(27):2800–2808
Rittigstein P, Torkelson J (2006) Polymer-nanoparticle interfacial interactions in polymer nanocomposites: confinement effects on glass transition temperature and suppression of physical aging. J Polymer Sci B—Polymer Phys 44(20):2935–2943
Moniruzzaman M, Winey K (2006) Polymer nanocomposites containing carbon nanotubes. Macromolecules 39(16):5194–5205
Erdem B, Hunsicker RA, Simmons GW, Sudol ED, Dimonie VL, El-Aasser MS (2001) XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation. Langmuir 17(9):2664–2669
Li W, Ni C, Lin H, Huang C, Shah S (2004) Size dependence of thermal stability of TiO2 nanoparticles. J Appl Phys 96(11):6663–6668
Zhai H, Wang L (2007) Probing the electronic structure and band gap evolution of titanium oxide clusters (TiO2)n-(n=1-10) using photoelectron spectroscopy. J Am Chem Soc 129(10):3022–3026
Okamoto M, Moritaa S, Taguchi H, Kima YH, Kotakaa T, Tateyamab H (2000) Synthesis and structure of smectic clay/poly(methyl methacrylate) and clay/polystyrene nanocomposites via in situ intercalative polymerization. Polymer 41(10):3887–3890
Dzumuzoric E, Jeremic K, Nedeljkovic JM (2007) In situ radical polymerization of methyl methacrylate in a solution of surface modified TiO2 and nanoparticles. Eur Polymer J 43(9):3719–3726
Dong W, Cogbill A, Zhang T, Ghosh S, Tian ZR (2006) Multifunctional, catalytic nanowire membranes and the membrane-based 3D devices. J Phys Chem B 110(34):16819–16822
Fan X, Lin L, Messersmith PB (2006) Surface-initiated polymerization from TiO2 nanoparticle surfaces through a biomimetic initiator: a new route toward polymer–matrix nanocomposites. Composites Sci Technol 66(9):1195–1201
Shirai Y, Kawatsura K, Tsubokawa N (1999) Graft polymerization of vinyl monomers from initiating groups introduced onto polymethylsiloxane-coated titanium dioxide modified with alcoholic hydroxyl groups. Prog Org Coating 36(4):217–224
Ding X, Wang Z, Han D, Zhang Y, Shen Y, Wang Z, Niu L (2006) An effective approach to synthesis of poly(methyl methacrylate)/silica nanocomposites. Nanotechnology 17(19):4796–4801
Jordan J, Jacob KI, Tannenbaum R, Sharaf MA, Jasiuk I (2005) Experimental trends in polymer nanocomposites. Mat Sci Eng: A 393(1–2):1–11
Li Q, Wu Y, Ma W, Xu R, Wu G, Yang W (2010) Synthesis of graft copolymers with polyisobutylene branch chains. Chin J Polym Sci 28(3):449–456
Vink H (1981) A new convenient method for the synthesis of poly (styrenesulfonic acid). Die Makromolekulare Chemie 182(1):279–281
Zarate RA, Fuentes S, Cabrera AL, Fuenzalida VM (2008) Structural characterization of single crystals of sodium titanate nanowires prepared by hydrothermal process. J Cryst Growth 310(15):3630–3637
Kim YI, Salim S, Huq M, Mallouk TE (1991) Visible-light photolysis of hydrogen iodide using sensitized layered semiconductor particles. J Am Chem Soc 113(25):9561–9563
Tian ZR, Yin YG, Suib SL, OYoung CL (1997) Effect of Mg2+ ions on the formation of todorokite type manganese oxide octahedral molecular sieves. Chem Mat 9(5):1126–1133
Tian ZR, Xia GG, Luo J, Suib SL, Navrotsky A (2000) Effects of water, cations, and structure on energetics of layer and framework phases, NaxMgyMnO2•nH2O. J Phys Chem B 104(20):5035–5039
Acknowledegments
The authors acknowledge the generous help from Mr. S. Michel at the Leibintz-institute for polymer research-Dresden-Germany on the contact angle measurements, and that from Prof. M. Macintosh’s lab at the University of Arkansas on the polymer synthesis. This work was partially supported by the Science and Technology Development Fund (STDF) (Project No. 1908), the US-Egypt Joint Research Grant, and the NSF-MRSEC and NSF-EPSCOR.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. F. Ghanem and R. L. Williams contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ghanem, A.F., Williams, R.L., Abdel Rehim, M.H. et al. Tuning a hydrophilic nanobelt’s crystal lattice for interface-tailored nanocompositing with a hydrophobic polymer. J Mater Sci 49, 7382–7390 (2014). https://doi.org/10.1007/s10853-014-8394-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8394-x