Abstract
PPY nanoparticles, as one of the important organic photothermal agents has been attracted great attention due to their good biocompatibility, high photothermal efficiency, and low cost. In order to further evaluate the size-dependent photothermal effect, PPY nanoparticles with two different sizes have been prepared with a facile method. The near infrared absorption and photothermal effect of the two size PPY nanoparticles were compared. The PPY nanoparticle with small size shows better photothermal effect at the same condition. The photostability, stability, and cytotoxicity in biologic system have also been investigated. In addition, the PPY nanoparticles with small size can kill the cancer cells effectively under the irradiation of 808 nm laser with a low-power density. These findings may provide better information for the application of the PPY nanoparticles on the photothermal ablation of cancer cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer is the leading cause of death in economically developed countries and the second leading cause of death in developing countries [1]. Laser-induced thermal therapy by converting optical energy into thermal energy can provide a minimally invasive and potentially more effective treatment alternative to conventional surgical resection [2]. A prerequisite for the development of the photothermal therapy is to gain strong light-absorbing materials as the photothermal agents, especially near-infrared (NIR, λ = 700–1100 nm) absorbing materials due to NIR laser is absorbed less by biological tissues and the typical penetration depth of the NIR light can be several centimeters in biological tissues [3]. Currently, several kinds of NIR photothermal agents have been developed, including gold [4], palladium [5] and silver [6] based metal nanostructure, copper-based semiconductor [7], and carbon [8, 9] based nanomaterials, and most of these photothermal agents show excellent effect of cancer therapy. However, there are still some limitations for these inorganic NIR absorbing materials. For example, the cost of the noble metal is very high, and nearly all of these inorganic NIR absorbing materials are non-biodegradable and usually remain in the body for long periods of time [10]. Thus, there is still a challenge for development biocompatible and efficient photothermal agent.
Compared with the inorganic nanomaterials, the organic nanomaterials show obvious advantages for the application in vivo, such as good biocompatible and easy biodegradation. Polypyrrole (PPY) is one of the well-known organic polymers and widely applied in bioelectronics and biomedical due to their unique properties, including high conductivity, outstanding stability, and good biocompatibility in vivo. Furthermore, PPY nanoparticles show strong absorption in the NIR region and have been demonstrated to be a good organic photothermal agent with high-photothermal efficiency, good biocompatible, and low cost [10–12]. However, the size of the PPY nanoparticles used in these report is different [10–12]. Recently, it has been demonstrated that PPY nanoparticles with different sizes show different cytotoxicity and innate immune responses [13]. Thus, it is necessary to investigate the photothermal effect of the PPY nanoparticles with different sizes.
In the present work, PPY nanoparticles with two different sizes have been synthesized by a facile one-step aqueous dispersion polymerization using polyvinyl alcohol (PVA) as a stabilizer and FeCl3 as an oxidant [10–12]. The size can be tuned by the molecular weight of the PVA. Then, the UV–Vis–NIR absorption and photothermal effect of these two size PPY nanoparticles have been investigated. HELA cells were used as the model cells to evaluate the photothermal ablation cancer cells of the PPY nanoparticles.
Experimental section
Materials
The pyrrole (98 %), iron(III) chloride hexahydrate (97 %), and PVA (molecular weight: 9000 and 130000, respectively) were purchased from Sigma-Aldrich. All the chemicals were used without further purification.
Preparation of PPY nanoparticles
PPY nanoparticles were synthesized by a modified literature procedure [10–12]. In a typical procedure for 50 nm PPY nanoparticles, FeCl3 (0.187 g) was added to 5 mL 7.5 % PVA (M w = 9000) solution and stirred for 1 h. Then, pyrrole monomer (34.6 μL) was added dropwise into the above solution at 3 °C. The chemical oxidation polymerization of pyrrole monomer was carried out for 4 h at 3 °C. For 75 nm PPY nanoparticles, it only need to take place of the PVA (M w = 9000) with PVA (M w = 130000). The dark-green PPY nanoparticles were collected by centrifugation.
Characterization of PPY nanoparticles
Sizes and morphologies of the PPY nanoparticles were determined by a transmission electron microscope (TEM) (JEOL 2100F). UV–Vis–NIR absorption spectra were measured on a Cary 5000 UV–Vis–NIR spectrophotometer using quartz cuvettes with an optical path of 1 cm. The temperature was recorded by an online-type thermocouple thermometer (DT-8891E Shenzhen Everbest Machinery Industry Co., Ltd, China) with an accuracy of ±0.1 °C.
Measurement of photothermal performance
An 808 nm NIR laser and an NMR tube containing an aqueous dispersion (0.6 mL) of the PPY nanoparticles at different concentrations (i.e., 25, 50, 75, 100, and 150 ppm) were used to measure the photothermal conversion performance. The concentration of the PPY nanoparticles was calculated in the deionized water first as the follow method. The weight of the aluminium foil box was tested and marked as m 1. Then, 1 mL PPY nanoparticles dispersed in deionized water were added into the aluminium foil box, and the deionized water was evaporated at 120 °C in an oven. At last, the weight of aluminium foil box with PPY nanoparticles was tested after cooling and marked as m 2. Thus, the concentration of PPY nanoparticles can be calculated by the equation (m 2 − m 1) g/mL. The power of the 808 nm semiconductor laser device (SFOLT Co. Ltd., China) can be adjusted from 0 to 0.55 W/cm2, and the laser diameter can be expanded to 5 cm. The online thermocouple thermometer (DT-8891E Shenzhen Everbest Machinery Industry Co., Ltd, China) with an accuracy of ±0.1 °C was used to record the temperature.
Cytotoxicity assay
The in vitro cytotoxicity was measured using the methyl thiazolyl tetrazolium (MTT) assay in human cervical carcinoma cell line HeLa. Cells growing in a log phase were seeded into 96-well cell-culture plate at 5 × 104/well in Dulbecco’s modified Eagle’s medium (DMEM) at 37 °C and in the presence of 5 % CO2 for 24 h, and then the cells were incubated with the PPY nanoparticles with different concentrations (i.e., 0, 25, 50, 75, 100, and 150 ppm, diluted in DMEM) for 12 and 24 h at 37 °C in the presence of 5 % CO2. Subsequently, 10 μL of MTT (5 mg/mL) was added to each well of the 96-well assay plate and incubated for more 4 h at 37 °C in the presence of 5 % CO2. After the addition of 10 % sodium dodecyl sulfate (SDS, 100 mL/well), the assay plate was allowed to stand at room temperature for 12 h. Multiskan MK3 monochromator-based multifunction microplate reader was used to measure the absorbance of each well with background subtraction at 492 nm. The tests were independently performed for three times.
Photothermal ablation of cancer cells in vitro
Hela cells were plated in 12-well plates at a density of 15000 cells/mL and via a 24 h prior treatment. Then, the phosphate buffer solution (PBS) dispersion containing the PPY nanoparticles with small size (75 ppm) as the treatment group and the PBS alone as control group were added into the wells. After 10 min irradiation of the 808 nm laser with a power density of 0.25 W/cm2, the cells were stained by 0.4 % trypan blue solution for 5 min. The images of the labeled cells were recorded immediately using an inverted biological microscope (XSP-18CE, Shanghai Changfang Optical Instrument Co., LTD). Each experiment was performed twice.
Result and discussion
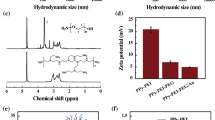
The morphology and size of the as-synthesized PPY nanoparticles was examined with a TEM (Fig. 1a, c). Typical TEM imaging revealed that both the resultant PPY nanoparticles were composed of mono-disperse nanoparticles possessing a narrow-size distribution and uniformly spherical in shape. The average diameter of the PPY nanoparticles synthesized with the PVA 9000 is about 50 nm (Fig. 1a), while the average diameter is increased to 75 nm (Fig. 1c) when the high-relative molecular weight polymer was used (PVA 13000). The size of the synthesized PPY nanoparticles in aqueous solution was measured by the dynamic light scattering (DLS) as shown in the Fig. 1b, d. The DLS result demonstrated that the synthesized PPY nanoparticles showed a narrow-size distribution even though in the aqueous solution. The size distributions were located at the 50 nm (Fig. 1b) and 75 nm (Fig. 1d) for the two nanoparticles synthesized with two different relative molecular weights of PVA, respectively, which were corresponded with the TEM results.
The optical properties of two-size PPY nanoparticles with the same concentration were studied by using UV–Vis–NIR spectroscopy (Fig. 2a). The spectra of two-size PPY nanoparticles are similar with what has been reported previously for the PPY nanoparticles, and they exhibit a strong absorption at the NIR region, which is the characteristic of the bipolaronic metallic state of PPY [10]. Importantly, the influence of the size on the absorption intensity of the as-synthesized PPY nanoparticles is very little as shown in Fig. 2a, which the PPY nanoparticles with large size is little stronger than the small size one at the NIR region with the same concentration. To demonstrate the size effectiveness of PPY nanoparticles on the photothermal conversion, we compared the temperature elevation of the PPY nanoparticles of the two sizes with the same concentration (150 ppm) under the irradiation of 808 nm laser with power density of 0.25 W/cm2. The temperature elevation of the 50 nm is 29.4 °C in 10 min, while that of the 75 nm PPY is 26.2 °C taking all other reaction parameters unaltered (Fig. 2b). It is evident that the NIR photothermal conversion efficiency of small-size PPY nanoparticles has been improved by ~12 % of magnitude compared to that of large size one. It is evident that the NIR photothermal conversion efficiency of PPY nanoparticles with small size is better than the one with large size. The high-photothermal effect of the PPY nanoparticles with small size should be attributed to the large-specific surface area of the PPY nanoparticle with small size which can transfer the heat from the PPY nanoparticles to water more quickly [14]. So, the small-size PPY nanoparticles will be used as the typical sample to investigate the properties of the PPY nanoparticles.
Giving that the concentration of photothermal agents is very important for the bio-application, the temperature elevation of PPY nanoparticle with small size dispersed in deionized water with different concentration (25–150 ppm) under the irradiation of 808 nm laser was investigated, and pure water was used as the control (Fig. 3a). The temperature of the solution can be precisely controlled from 21 to 51 °C by varying the concentration of the PPY nanoparticles. Figure 3b showed the temperature change over 10 min versus the concentration of the PPY nanoparticles, which calculated from the Fig. 3a. From Fig. 3b, it can be seen that the temperature change goes up dramatically with the increase of PPY nanoparticle concentration. These results indicate that the PPY nanoparticles with small size can convert the photo energy to heat efficiently and transfer to water quickly. It can be used as excellent agents for photothermal ablation cancer cells.
Photobleaching is the disadvantage for the most of the organic photothermal agents, especially for the small molecular weight one [2]. In order to investigate the photostability of the PPY nanoparticles, the constant photothermal conversion behavior of the PPY nanoparticles dispersed in water was tested by treating it with the laser on-and-off cycles. As shown in Fig. 4a, the temperature elevation of the last cycles is the same with the first one even after six cycles (the PPY nanoparticles was irradiated for 10 min in each cycle). Figure 4b is the photograph of the PPY nanoparticles dispersion before and after six cycles of laser on-and-off treatment. There is no obvious color change for the PPY nanoparticles dispersion after the test. Comparison of the TEM images of the PPY nanoparticles which correspond to before (Fig. 4c) and after (Fig. 4d) six cycles of laser on-and-off treatment showed that there was no change in shape and size of the PPY nanoparticles. These results demonstrated that the PPY nanoparticles shown very good photostability compared with conventional organic photothermal agents with small molecular weight reported previously [2].
a Temperature monitoring of a PPY nanoparticles aqueous suspension (150 ppm) during six successive cycles of an on-and-off laser. The laser (808 nm, 0.25 W/cm2) was on for 10 min in each cycle. b Photograph of the PPY nanoparticles aqueous suspension before and after the laser irradiation for six cycles and corresponding TEM images before (c) and after (d) laser irradiation
The stability of materials in biologic system is also very important for bio-application [15]. The PBS was simulated biologic system to test the stability of the PPY nanoparticles with small size. Figure 5a is the UV–Vis–NIR absorption spectrum of the PPY nanoparticles dispersed in PBS with different concentration after settling for 1 week. The absorption peak of the PPY nanoparticles dispersed in PBS is similar with the deionized water (Fig. 2a blue line). The absorption strength of the PPY nanoparticles dispersed in PBS at 808 nm versus concentration is shown in Fig. 5b. The absorption strength for different concentrations still linearly increased with the particle concentration even after settling for 1 week, which means no precipitate formed in this period. Because the concentration of the PPY nanoparticles will be changed and the absorption intensity will not be linearly increased, if the solution gives a precipitate. So, the linearly increased with the particle concentration can demonstrate the good dispersibility of PPY nanoparticles in PBS. The photograph (inset of Fig. 5b) is the PPY nanoparticles dispersion in PBS which left to stand for 1 week. There is no precipitate found at the bottom of the tube, which further conforms the good dispersibility of PPY nanoparticles in PBS. The good dispersibility of PPY nanoparticles in PBS indicated the good stability in biologic system.
a UV–Vis–NIR absorption spectra of the PPY nanoparticles dispersed in PBS with different concentrations collected 1 week after the dispersion. b Linear fitting of the absorption strength of PPY nanoparticles suspension at 808 nm versus the particle concentrations. Inset photograph of the PPY nanoparticles dispersed in PBS after 1 week
For one kind of materials used in biological system, the ideal photothermal agent should be nontoxic or low-toxic [16]. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay method and human cervical carcinoma cell line Hela were used to evaluate the cytotoxicity of the PPY nanoparticles with small size. In order to get a reliable result, the cytotoxicity measurement experiment was carried out for three groups in parallel and the average values are shown in the Fig. 6. According to the proliferation of the cells in Fig. 6, there are no obvious differences without and with the presence of PPY nanoparticles with concentrations of 25–100 ppm, and cellular viability was estimated to be higher than 90 % even after 24 h, which can be considered to have a very low cytotoxicity even at a high concentration (150 ppm). The very low cytotoxicity of the PPY nanoparticles suggested that it is an ideal bio-compatible photothermal agent.
To verify our hypothesis, the photothermal ablation capability from PPY nanoparticles with small size was investigated in vitro using the HELA cells derived from human cervical carcinoma cell line as a model. As shown in (Fig. 7), nearly all the cells are dead in the group treated with both PPY nanoparticles with small size and the laser irradiation (Fig. 7d), while the cell treated without the laser and PPY nanoparticles with small size (Fig. 7a), with PPY nanoparticles with small size (Fig. 7b), or with laser alone shows no apparent cell death (Fig. 7c). Therefore, this PPY nanoparticle with small size has great potentials to be used as an ideal photothermal-coupling agent for photothermal ablation of specific targets such as in vivo tumor tissues.
Conclusions
In summary, uniformly water-soluble PPY nanoparticles with two sizes were synthesized by a facile method. The NIR absorption and photothermal effect of the PPY nanoparticle with two sizes were investigated. With same condition, the absorption of the two-size PPY nanoparticle shows no obvious difference, while the photothermal effect of the PPY nanoparticles with small size is better than the one with large size. The PPY nanoparticles with small size show good photostability, bio-stability, and low cytotoxicity. Furthermore, it can efficiently kill the cancer cells under the irradiation of 808 nm laser with a very low-power density of 0.25 W/cm2 over 10 min. These results demonstrate the great potential application of PPY nanoparticles with small size in photothermal ablation of in vivo tumor tissues.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Tian Q, Jiang F, Zou R, Liu Q, Chen Z, Zhu M, Yang S, Wang J, Wang J, Hu J (2011) Hydrophilic Cu9S5 nanocrystals: a photothermal agent with a 25.7 % heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano 5:9761–9771
Chen WR, Adams RL, Carubelli R, Nordquist RE (1997) Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer Lett 115:25–30
Skrabalak SE, Chen J, Au L, Lu X, Li X, Xia Y (2007) Gold nanocages for biomedical applications. Adv Mater 19:3177–3184
Huang X, Tang S, Mu X, Dai Y, Chen G, Zhou Z, Ruan F, Yang Z, Zheng N (2011) Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat Nano 6:28–32
Xu R, Ma J, Sun X, Chen Z, Jiang X, Guo Z, Huang L, Li Y, Wang M, Wang C, Liu J, Fan X, Gu J, Chen X, Zhang Y, Gu N (2009) Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Res 19:1031–1034
Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW, Korgel BA (2011) Copper selenide nanocrystals for photothermal therapy. Nano Lett 11:2560–2566
Robinson JT, Tabakman SM, Liang Y, Wang H, Sanchez Casalongue H, Vinh D, Dai H (2011) Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J Am Chem Soc 133:6825–6831
Yang K, Zhang S, Zhang G, Sun X, Lee S-T, Liu Z (2010) Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett 10:3318–3323
Zha Z, Yue X, Ren Q, Dai Z (2013) Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv Mater 25:777–782
Chen M, Fang X, Tang S, Zheng N (2012) Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy. Chem Commun 48:8934–8936
Yang K, Xu H, Cheng L, Sun C, Wang J, Liu Z (2012) In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv Mater 24:5586–5592
Kim S, Oh W-K, Jeong YS, Hong J-Y, Cho B-R, Hahn J-S, Jang J (2011) Cytotoxicity of, and innate immune response to, size-controlled polypyrrole nanoparticles in mammalian cells. Biomaterials 32:2342–2350
Roper DK, Ahn W, Hoepfner M (2007) Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J Phys Chem C 111:3636–3641
Tian Q, Hu J, Zhu Y, Zou R, Chen Z, Yang S, Li R, Su Q, Han Y, Liu X (2013) Sub-10 nm Fe3O4@Cu2–xS core–shell nanoparticles for dual-modal imaging and photothermal therapy. J Am Chem Soc 135:8571–8577
Tian Q, Tang M, Sun Y, Zou R, Chen Z, Zhu M, Yang S, Wang J, Wang J, Hu J (2011) Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv Mater 23:3542–3547
Acknowledgements
The authors gratefully acknowledge the financial support by the Shanghai Municipal Nature Science Foundation (Grant No. 13ZR1433300), the Shanghai Committee of Science and Technology, China (Grant No. 11JC1410400), the Shanghai Jiao Tong University Biomedical Engineering (Physical) Crossover Foundation (Grant No. YG2011MS57), the National Natural Science Foundation of China (Grant Nos. 21171035 and 51302035), the Key Grant Project of Chinese Ministry of Education (Grant No. 313015), the PhD Programs Foundation of the Ministry of Education of China (Grant Nos. 20110075110008 and 20130075120001), the National 863 Program of China (Grant No. 2013AA031903), the Science and Technology Commission of Shanghai Municipality (Grant No. 13ZR1451200), the Fundamental Research Funds for the Central Universities, Program for Changjiang Scholars and Innovative Research Team in University (Grant No. IRT1221), the Shanghai Leading Academic Discipline Project (Grant No. B603), and the Program of Introducing Talents of Discipline to Universities (No. 111-2-04).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Q., Wang, J., Lv, G. et al. Facile synthesis of hydrophilic polypyrrole nanoparticles for photothermal cancer therapy. J Mater Sci 49, 3484–3490 (2014). https://doi.org/10.1007/s10853-014-8061-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8061-2