Abstract
The microstructure and thermal stability of the Mg97Y2Zn1 (at.%) alloy, modified with the addition of 0.5 at.% of gadolinium or neodymium, have been examined by synchrotron radiation diffraction during in situ differential scanning calorimetry. The microstructure of the three alloys consists of magnesium dendrites with the Long Period Stacking Ordered (LPSO) phase at interdendritic regions. Rare-earth atoms substitute yttrium atoms in the LPSO phase, promoting the formation of the 14H structure. Lattice parameters of the LPSO do not change significantly with the rare-earth addition. However, they reduce the melting point of the LPSO phase, especially in the case of neodymium addition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnesium alloys combining yttrium/rare-earth (Y, Gd, Nd, Dy, etc.) elements and transition metals (Zn, Cu, and Ni) are effectively strengthened by the presence of Long Period Stacking Ordered (LPSO) structures [1–8]. They can combine high strength with acceptable ductility and enhanced creep resistance. LPSO-phases are solid solutions of yttrium or rare-earth elements and transition metals in the magnesium lattice, where these atoms are arranged periodically in the magnesium basal planes forming ordered structures [9–12]. Different LPSO crystal structures have been reported, i.e., 6H, 10H, 14H, 18R, and 24R [13–15] depending on the thermal history of the material. The 18R structure is observed mainly in the as-cast condition. However, this structure is not stable at high temperature and is gradually replaced by a 14H structure [11]. Recently, Egusa and Abe [12] have proposed crystal/order structural models for 18R and 14H phases formed in Mg–Zn–RE alloys. These phases can be rationalized by space groups C2/m, P3112, or P3212 for the 18R type and P63/mcm for the 14H type. The characteristic-ordered features are well represented by local Zn6RE8 clusters, which are embedded in the fcc-stacking layers in accordance with the L12 type short-range order.
A Mg97Y2Zn1 (at.%) alloy produced by warm extrusion of rapid solidified ribbons exhibited high-yield strength, about 610 MPa, with 5 % of elongation at room temperature [1]. Recently, Yamasaki et al. [4] have developed extruded Mg97Y2Zn1 (at.%) alloys that combine high-yield stress (around 350 MPa) and acceptable ductility (around 8 %) by controlling the cooling rate during the casting process and the extrusion parameters.
Optimization of the microstructure by developing fine lamella inside the magnesium grains can be achieved through an appropriate thermal treatment, resulting in additional strengthening [8, 16, 17]. At room temperature, the hardening induced by these long plates is small. This fact is attributed to the crystallographic orientation of the precipitates within the magnesium matrix. Since they are located in basal planes, they have a low-hardening effect on the basal slip system [18]. However, at high temperature, where nonbasal slip systems are active, the lamellar structure increases the creep resistance of these alloys [8]. In a recent review about precipitation and hardening in magnesium alloys [19], Nie concluded that precipitate plates formed on prismatic planes provide the most effective barrier to gliding dislocations and propagating twins in the magnesium matrix. The formation of this kind of precipitates has been reported in Mg–Y–RE systems, such as Mg–Y–Nd, Mg–Gd, Mg–Gd–Nd, etc. Moreover, Nie suggested that a higher strength can be achieved if a high density of strong plate-shaped precipitates with prismatic and basal habit planes and with a high-aspect ratio can be introduced. Recently, it has been reported that the addition of rare-earth elements to the Mg–Y–Zn system containing LPSO phases promote the formation of plates in the prismatic and basal planes, preserving the LPSO phase in some conditions, and considerably increasing the strength of Mg–Y–RE–Zn (RE = Gd and Nd) alloys at room temperature [20–22].
The presence of additional elements can modify the crystallography and thermal stability of the LPSO phase. Therefore, this article explores the influence of the addition of 0.5 at.% of Gd and 0.5 at.% of Nd to the Mg97Y2Zn1 (at.%) alloy.
Experimental procedure
Three alloys with nominal compositions Mg97Y2Zn1 (at.%), Mg96.5Y2Zn1Gd0.5 (at.%), and Mg96.5Y2Zn1Nd0.5 (at.%) were prepared by melting high-purity elements Mg and Zn and Mg–22 %Y (wt%), Mg–20 %Gd (wt%), and Mg–22 %Nd (wt%) master alloys in an electric resistance furnace. The three alloys were cast in a 12-mm diameter cylindrical steel mold. For comparative purposes, a fully LPSO alloy with composition Mg88Y8Zn4 (at.%) was also prepared in an induction melting furnace using a graphite tube coated with boron nitride in argon atmosphere. The fully LPSO alloy was homogenized at 350 °C for 24 h.
Microstructural characterization of the alloys was carried out using optical (OM), scanning (SEM) and transmission electron microscopy (TEM). Specimens for TEM observation were prepared by means of electrolytic polishing using a reactive mixture of 25 % nitric acid and 75 % methanol at −20 °C and 20 V, respectively. Then, ion milling at liquid nitrogen temperature was used to remove the fine oxide film formed on the surface during electrolytic polishing.
The thermal stability of the three alloys was monitored by means of DSC experiments using heating/cooling rates of 10 K min−1 under argon atmosphere in the Mettler Toledo 822 DSC equipment. The samples were subjected to one DSC heating/cooling cycle between RT and 570 °C.
Synchrotron radiation diffraction was performed at the P07—HEMS beamline of PETRA III, at the DeutschesElektronen-Synchrotron (DESY) during in situ DSC experiments. The samples were encapsulated in stainless steel crucibles during the measurement, using an empty crucible as reference. The DSC unit was placed inside the coil of a Bähr 805A/D dilatometer. The dilatometer and the DSC unit are modified for synchrotron experiments. Two Kapton windows on the sides of the chamber and a hole in the DSC pan allow the high-energy X-ray beam to reach the sample and the detector without disturbance. The measurements were performed in argon flow. The diffraction patterns were recorded using an exposure time of 1 s by means of a Perkin-Elmer XRD 1622 flat panel detector with an array of 20482 pixels, with an effective pixel size of 200 × 200 μm2. The beam energy was 100 keV, corresponding to a wavelength of 0.0124 nm. LaB6 was used as a reference to calibrate the acquired diffraction spectra. The detector-to-sample distance was 1918.95 mm. Conventional line profiles were obtained by azimuthal integration of the Debye–Scherrer rings. The heating/cooling rate of the DSC was 40 and 10 K min−1 below and above 400 °C, respectively. Similarly to the laboratory DSC scans, the samples were subjected to one DSC cycle between RT and 560 °C.
Results and discussion
Figure 1a–c shows the microstructure of the MgY2Zn1(RE0.5) alloys in the as-cast condition. The microstructure consists of magnesium dendrites with the LPSO phase distributed at interdendritic regions. The volume fraction of LPSO phase is similar for the three alloys, about 22 vol%. The Mg88Y8Zn4 (at.%) alloy consists of long LPSO laths with a small volume fraction of magnesium islands (Fig. 1d).
Figure 2 a–h shows the Debye–Scherrer rings obtained at RT for the alloys in the as-cast condition. It is interesting to note that diffuse scattering (streaking) is observed in the case of the LPSO phase in the MgY2Zn1(RE0.5) alloys. This is especially marked for the smaller diffraction ring that corresponds to (0003) reflex of the 18R LPSO phase. Okuda et al. [23], using SAXS, reported diffuse streaks connecting diffracted spots suggesting that 18R and 10H structures coexist in a coherent way in the same grains. However, in this case, these diffuse streaks are not observed connecting reflexes from other LPSO structures. On the other hand, similar diffuse streaking has been observed in diffraction patterns using TEM. Li et al. [24] showed diffuse streaking in the [0001] direction due to the formation of stacking faults (SFs) in the (0001) basal plane of deformed pure magnesium. Garces et al. [8] also observed diffuse streaking along the [0002] direction in the magnesium grains of MgY2Zn1 alloys heat treated at high temperature. This diffuse streaking was related to SFs in the basal plane due to the formation of long LPSO precipitates having the 14H structure. The fact that the streaking is radial to the diffraction ring indicates that the SFs are located parallel to the (0003) planes, namely in the c direction of the 18R LPSO lattice.
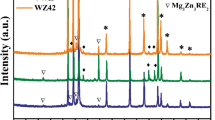
The integration of the Debye–Scherrer rings to obtain the diffraction patterns as a function of 2θ has been carried out using the software Fit2D [25]. As an example, Fig. 3 shows the indexed Debye–Scherrer rings and the 2θ diffraction patterns for the MgY2Zn1 alloy. Figure 4 shows the integrated diffraction patterns obtained at room temperature for the four alloys in the as-cast condition together with the theoretical diffraction reflections of Mg and those of the 18R and 14H LPSO phase. The diffraction pattern of the 18R and 14H crystal structures were simulated using Carine 3.1 [26] according to the structure described in [12]. The 18R type and 14H type crystal structures belong to the space groups P3212 and P63/mcm, respectively. It can be seen that the crystal structure of the LPSO phase in the four as-cast alloys corresponds mainly to the 18R structure.
Lattice parameters of LPSO and magnesium phases can be calculated from the diffraction patterns of Fig. 4. In all studied alloys, the use of a Rietveld fit is difficult due to the large overlapping exhibited by several reflexes of Mg and LPSO structures. Therefore, isolated peaks from each phase have been individually fitted using the software PeakFit [27]. Table 1 shows a and c values for the LPSO and magnesium phases, respectively. The lattice parameters of the LPSO phase are similar in all the cases and are in agreement with the literature [12]. The fitting errors in MgY2Zn1(RE0.5) alloys are larger than for the MgY8Zn4 alloy because the intensity of diffracted peaks corresponding to the LPSO phase is smaller and the diffracted peaks are broader due to the diffusive scattering (Fig. 2e–g). The lattice parameter a of Mg shows an increase with respect to pure Mg (a = 0.32089 nm and c = 0.52101 nm [28]). The magnesium phase in the MgY2Zn1 (RE0.5) alloys contains a small amount of yttrium in solid solution which is reported to increase a, i.e., reducing the c/a ratio [29, 30].
As described above, the diffuse scattering results in a very broad (0003) diffraction peak of the 18R structure, located at 2θ = 0.44°. Furthermore, overlapping with a second peak at smaller angles seems to take, especially for the Mg97Y2Zn1Nd0.5 alloy. This may indicate the coexistence of 18R and 14H LPSO in the as-cast alloys. Figure 5a shows a detail of the lamellar structure corresponding to the LPSO phase in the as-cast Mg97Y2Zn1Nd0.5 alloy. The contrast variation in the SEM image can be related to composition or crystallographic heterogeneities. Figure 5b provides a two-beam bright-field image for the LPSO phase using g = 0002α diffraction vector in the \( \left\langle {11\overline{2} 0} \right\rangle_{\alpha}\) zone axis in the Mg97Y2Zn1Nd0.5 alloy. The18R and 14H crystal structures can be identified through the selected area electron diffraction pattern (SAED) in the \( \left\langle {11\overline{2} 0} \right\rangle_{\alpha}\) zone axis or by measuring the fringe spacing in the[0002]α direction formed when the g = 0002 is excited. The coexistence of 18R and 14H structures with a fringe spacing of about 1.6 and 1.8 nm, respectively, can be observed. This fact can be also observed in the SAED in the \( \left\langle {11\overline{2} 0} \right\rangle_{\alpha}\) zone axis where the diffracted spots of both structures are overlapped. Moreover, the SAED pattern shows weak streaks along [0001] direction and are visible through the ±1/2{1100} positions. Zhu et al. [11] indicated that the presence of these weak streaks provides important information on the ordered arrangement of Y and Zn in the 18R and 14H structures.
a Secondary electron image of the microstructure of the as-cast Mg97Y2Zn1Nd0.5 alloy. b Bright field TEM image of the LPSO phase in the as-cast Mg97Y2Zn1Nd0.5 alloy [B = \( \left\langle {11\overline{2} 0} \right\rangle \) and g = (0002)]. c Selected area diffraction pattern of the Mg97Y2Zn1Nd0.5 alloy in the \( \left\langle {11\overline{2} 0} \right\rangle \) zone axis
Table 2 shows the composition of the LPSO phase determined using energy dispersive spectroscopy (EDS) for the three MgY2Zn1(RE0.5) alloys in the as-cast condition and after the DSC scan. The composition of the LPSO phase in the Mg97Y2Zn1 (at.%) alloy is similar to that reported in a previous study [11]. Gadolinium and neodymium additions have two different effects on LPSO phase composition. On one hand, zinc content slightly decreases with respect to LPSO formed in the Mg97Y2Zn1 alloy. On the other hand, yttrium content decreases up to ~5 at.%, while the rare-earth content is around 1.5 at.% in both alloys in as-cast condition. The sum of yttrium and rare-earth concentration in quaternary alloys is equal to the yttrium content in the ternary alloy, indicating that rare-earth atoms substitute yttrium atoms in the LPSO structure. Thus, an increase in the volume fraction of the LPSO phase in the Mg97Y2Zn1Gd0.5 and Mg97Y2Zn1Nd0.5 alloys may be expected due to the excess of yttrium. However, the volume fraction of the LPSO phase is similar in the three alloys (about 22 vol%) suggesting that the maximum volume fraction of the LPSO phase in the alloys is fixed by the zinc content. Therefore, the excess of yttrium and rare-earth elements could favor the formation of new phases.
Figure 6 shows laboratory DSC scans during heating and cooling for the three Mg97Y2Zn1(RE)0.5 alloys. It is important to note that the use of the term “laboratory” is used to distinguish from the DSC scans obtained in the in situ experiments. An endothermic peak is observed during heating and one or two exothermic peaks during cooling depending on the alloy. The endothermic and exothermic reactions are displaced to lower temperatures with the additions of rare-earth elements. Neodymium results in a larger temperature displacement than gadolinium. During heating, the rare-earth-containing alloys show only one endothermic reaction, but two clear exothermic peaks can be observed during cooling. The separation between the exothermic reactions is larger for the alloy with Nd than for that with Gd addition. Figure 7a shows synchrotron diffraction patterns for the Mg97Y2Zn1 (at.%) alloy at 400 °C during heating, at 560 °C, (the temperature at the end of the endothermic peak) and at 400 °C during cooling. At 400 °C during heating, reflections corresponding to the magnesium matrix, the LPSO phase with the 18R crystal structure and the stainless steel pan (SP) are observed. At 560 °C, right after the endothermic peak, the reflections of the 18R crystal structure disappear, remaining exclusively reflections from the magnesium phase and the SP. At 400 °C, during cooling, diffraction peaks corresponding to the LPSO phase reappear, but now with coexisting 18R and 14H structures. This indicates that the endothermic and exothermic reactions are related to melting and solidification of the LPSO phase, respectively. The addition of the rare-earth elements decreases the melting point of the LPSO phase, as it is evidenced in Fig. 6 by the displacement of the endothermic peaks of the Mg97Y2Zn1Gd0.5 and Mg97Y2Zn1Nd0.5 alloys to lower temperatures in comparison with the Mg97Y2Zn1 alloy. Since the melting points of yttrium, gadolinium, and neodymium are 1526, 1311, and 1010 °C [28], respectively, the highest melting point for the LPSO phase should be expected for the Mg97Y2Zn1, followed by the Gd-containing and Nd-containing alloys, as observed in DSC scans. This agrees with the experimental data (end temperature of the endothermic peak: 561, 556, and 542 °C for Mg97Y2Zn1, Mg97Y2Zn1Gd0.5, and Mg97Y2Zn1Nd0.5, respectively).
a Synchrotron diffraction patterns at 400 and 560 °C during heating and at 400 °C during cooling for the Mg97Y2Zn1 alloy. b Synchrotron diffraction patterns at 400 °C during heating, after the endothermic peak during heating and at 400 °C during cooling for the Mg97Y2Zn1,Mg97Y2Zn1Gd0.5, Mg97Y2Zn1Nd0.5, and Mg97Y8Zn4 alloys
Figure 7b shows the synchrotron diffraction patterns at 400 °C during heating, at the highest temperature above the endothermic peak and at 400 °C during cooling for the four alloys in the 2θ range of 0.2°–0.75°. In this 2θ range the diffraction peaks of the 18R and 14H crystallographic structures do not overlap considerably. The 18R structure is exclusively present in the alloys in as-cast condition since the cooling rate during casting was sufficiently fast to prevent formation of 14H structure [11]. However, while 18R and 14H structures coexist after the exothermic reaction during cooling in the three MgY2Zn1(RE)0.5 alloys, the fully LPSO alloy only shows the 18R structure. The presence of the rare earth in solid solution in the LPSO phase has a strong influence in its crystal structure during its formation in the course of solidification. Figure 8 shows the diffraction patterns at low-diffraction angle for the four studied alloys during cooling at the initial stage of the solidification of the LPSO phase. It must be pointed out that there is a difference of about +15 °C between the laboratory DSC and the in situ synchrotron diffraction experiments. This may arise due to the position of the thermocouple in the DSC unit of the Bähr dilatometer. This difference provokes a displacement of the temperature at which the reactions are measured but it does not influence the results. The temperature measured with the laboratory DSC equipment is more reliable and it must be considered as the correct one. Synchrotron diffraction data demonstrates that the 18R structure is formed first and then followed by the 14H one in the case of the MgY2Zn1 and MgY2Zn1Gd0.5 alloys. However, in the MgY2Zn1Nd0.5 alloy, the 14H structure is rapidly developed and the intensity of the diffracted peak is even higher than that of the 18R structure. This is in agreement with the microstructure found in the as-cast condition, where the 14H structure is formed during solidification (Figs. 4, 5). These results indicate that the exothermic peak during cooling corresponds in fact to the overlapping formation of the 14H and 18R structures. However, the 18R structure is unstable and transforms into the 14H structure during heat treatments above 400 °C [11]. Therefore, the 14H structure can be expected for sufficiently slow cooling rates. However, recent study by Grobner et al. [31], which examine a phase diagram for LPSO and related phases in the Mg-rich corner of the ternary alloys, suggests a stable region in which 18R and 14H structures can coexist, as observed in this study. It is interesting to point out that in the case of the fully LPSO alloy, no 14H structure has been observed after cooling in the present study. The formation of 14H structure has been reported in the same alloy during a thermal treatment of 525 °C for 3 days [32]. Since transformation from 18R to 14H structures is slow, it is expected that lower cooling rates are required to observe the appearance of the diffracted peaks corresponding to the 14H structure in the fully LPSO alloy.
Figure 9 shows the microstructure of the Mg97Y2Zn1Nd0.5 alloy after the DSC scan. In the three alloys, the microstructure consists of magnesium and LPSO phases. The formation of a lamellar structure within the magnesium matrix is also found, identical to that reported previously in the Mg97Y2Zn1 alloy [8, 16, 17, 33]. The chemical composition of the LPSO phase determined by EDS after the DSC scan is very close to that in as-cast condition for the Mg97Y2Zn1 and Mg97Y2Zn1Gd0.5 alloys (see Table 2). However, the Nd content in the LPSO phase in the Mg97Y2Zn1Nd0.5 alloy falls almost 50 %. Moreover, a new quaternary intermetallic phase has formed during cooling. This phase appears at lower temperatures than the LPSO phase as evidenced by the last exothermic peak at about 505 °C for the Mg97Y2Zn1Nd0.5 alloy in Fig. 6. Its composition is also listed in Table 2. The crystallography of this new phase is now under research. It has been reported that the LPSO phase forms in Mg97RE2Zn1 alloys when yttrium and gadolinium are added but not in the case of Nd [5]. Therefore, the amount of neodymium dissolved in the LPSO phase is lower, 0.8 at.%, than that of gadolinium. The excess of neodymium is free to promote the formation of the new intermetallic phase found in this study.
Conclusions
The effect of the addition of 0.5 at.% of RE (Gd and Nd) on the microstructure and thermal stability of the LPSO phase in an Mg97Y2Zn1 (at.%) alloy has been studied. The microstructure of the as-cast alloy does not change significantly with the addition of Nd or Gd and consists of magnesium dendrites with the 18R LPSO phase distributed at interdendritic regions. RE atoms substitute yttrium atoms in the LPSO phase in the as-cast condition and modified the crystallography of the LPSO phase, especially in the case of the addition of Nd which promotes the formation the 14H structure. Moreover, in this alloy, the LPSO phase is not stable and a new intermetallic phase is formed after the 14H and 18R LPSO phases during cooling from 570 °C at 10 K min−1. RE addition decreases the melting point of the LPSO phase. The lower the melting point of the RE, the lower the melting point of the LPSO phase.
References
Inoue A, Kawamura Y, Matsushita M, Hayashi K, Koike J (2001) Novel hexagonal structure and ultrahigh strength of magnesium solid solution in the Mg–Zn–Y system. J Mater Res 16:1894–1900
Kawamura Y, Kasahara T, Izumi S, Yamasaki M (2006) Elevated temperature Mg97Y2Cu1 alloy with long period ordered structure. Scripta Mater 55:453–456
Hagihara K, Kinoshita A, Sugino Y, Yamasaki M, Kawamura Y, Yasuda HY, Umakoshi Y (2010) Effect of long-period stacking ordered phase on mechanical properties of Mg97Zn1Y2 extruded alloy. Acta Mater 58:6282–6293
Yamasaki M, Hashimoto K, Hagihara K, Kawamura Y (2011) Effect of multimodal microstructure evolution on mechanical properties of Mg–Zn–Y extruded alloy. Acta Mater 59:3646–3658
Kawamura Y, Yamasaki M (2007) Formation and mechanical properties of Mg97Zn1RE2 alloys with long period stacking ordered structure. Mater Trans 48:2986–2992
Garces G, Pérez P, Gonzalez S, Adeva P (2006) Development of long-period ordered structures during crystallization of amorphous Mg80TM10Y10. Int J Mater Res 4:404–408
Mi SB, Jin QQ (2013) New polytypes of long-period stacking ordered structures in Mg–Co–Y. Scripta Mater 68:635–638
Garces G, Oñorbe E, Dobes F, Pérez P, Antoranz JM, Adeva P (2012) Effect of microstructure on creep behaviour of cast Mg97Y2Zn1 (at.%) alloy. Mater Sci Eng A 539:48–55
Luo ZP, Zhang SQ (2000) High-resolution electron microscopy on the X–Mg12ZnY phase in a high strength Mg–Zn–Zr–Y magnesium alloy. J Mater Sci Lett 19:813–816
Abe E, Kawamura Y, Hayashi K, Inoue A (2002) Long-period ordered structure in a high-strength nanocrystalline Mg-1 at% Zn-2 at% Y alloy studied by atomic-resolution Z-contrast STEM. Acta Mater 50:3845–3857
Zhu YM, Morton AJ, Nie JF (2010) The 18R and 14H long-period stacking ordered structures in Mg–Y–Zn alloys. Acta Mater 58:2936–2947
Egusa D, Abe E (2012) The structure of long period stacking/order Mg–Zn–RE phases with extended non-stoichiometry ranges. Acta Mater 60:166–178
Chino Y, Mabuchi M, Hagiwara S, Iwasaki H, Yamamoto A, Tsubakino H (2004) Novel equilibrium two phase Mg alloy with long-period ordered structure. Scripta Mater 51:714–774
Matsuda M, Ii S, Kawamura Y, Ikuhara Y, Nishida M (2005) Variation of long-period stacking order structures in rapidly solidified Mg97Zn1Y2 alloy. Mater Sci Eng A 393:269–274
Abe E, Ono A, Itoi T, Yamasaki M, Kawamura Y (2011) Polytypes of long-period stacking structures synchronized with chemical order in a dilute Mg–Zn–Y alloy. Philos Mag Lett 91:690–696
Nie JF, Oh-ishi K, Gao X, Hono K (2008) Solute segregation and precipitation in a creep-resistant Mg–Gd–Zn alloy. Acta Mater 56:6061–6076
Yamasaki M, Sasaki M, Nishijima M, Hiraga K, Kawamura Y (2007) Formation of 14H long period stacking ordered structure and profuse stacking faults in Mg–Zn–Gd alloys during isothermal aging at high temperature. Acta Mater 55:6798–6805
Geng J, Chun YB, Stanford N, Davies CHJ, Nie JF, Barnett MR (2011) Processing and properties of Mg–6Gd–1Zn–0.6Zr: Part 2. Mechanical properties and particle twin interactions. Mater Sci Eng A 528A:3659–3665
Nie JF (2012) Precipitation and hardening in magnesium alloys. Metall Mater Trans A 43:3891–3939
Honma T, Ohkubo T, Kamado S, Hono K (2007) Effect of Zn additions on the age-hardening of Mg–2.0Gd–1.2Y–0.2Zr alloys. Acta Mater 55:4137–4150
Yin DD, Wang QD, Gao Y, Chen CJ, Zheng J (2011) Effects of heat treatments on microstructure and mechanical properties of Mg–11Y–5Gd–2Zn–0.5Zr (wt%). J Alloys Compd 509:1696–1704
Lee JB, Sato K, Konno TJ, Hiraga K (2011) Complex precipitates with long periodic stacking (LPS) phase and precipitation behaviors in the Mg97Zn1Y1.5Nd0.5 alloy by age-annealing. Intermetallics 19:1096–1101
Okuda H, Horiuchi T, Tsukamoto T, Ochiai S, Yamasaki M, Kawamura Y (2013) Evolution of long-period stacking ordered structures on annealing as-cast Mg85Y9Zn6 alloy ingot observed by synchrotron radiation small-angle scattering. Scripta Mater 68:575–578
Li B, Yan PF, Sui ML, Ma E (2010) Transmission electron microscopy study of stacking faults and their interaction with pyramidal dislocations in deformed Mg. Acta Mater 58:173–179
Hammersley AP, Svensson SO, Hanfland M, Fitch AN, Häusermann D (1996) Two-dimensional detector software: from real detector to idealised image or two-theta scan. High Press Res 14:235–248
caRine crystallography software version 3.1 Cyrille Boudias and Daniel Monceau. http://carine.crystallography.pagespro-orange.fr/
PeakFit software version 4.11 AISN Software Inc. http://www.sigmaplot.com/products/peakfit/peakfit.php
Villars P, Calvert LD (1985) Pearson′s handbook of crystallographic data for intermetallic Phases. American Society for Metals, Metal Park
Hanawa S, Sugamata M, Kaneko J (1997) Structures and mechanical properties of rapidly solidified Mg–Y based alloys. Mater Sci Eng A 226–228:861–866
Agnew SR, Yoo MH, Tome CN (2001) Application of texture simulation to understanding mechanical behavior of Mg and solid solution alloys containing Li or Y. Acta Mater 49:4277–4289
Grobner J, Kozlov A, Fang XY, Geng J, Nie JF, Schimdt-Fetzer R (2012) Phase equilibria and transformations in ternary Mg-rich Mg–Y–Zn alloys. Acta Mater 60:5948–5962
Hagihara K, Sugino Y, Fukusumi Y, Umakoshi Y, Nakano T (2011) Plastic deformation behavior of Mg12ZnY LPSO-phase with 14H-typed structure. Mater Trans 52:1096–1103
Oñorbe E, Garces G, Perez P, Adeva P (2012) Effect of the LPSO volume fraction on the microstructure and mechanical properties of Mg–Y2x–Znx alloys. J Mater Sci 47:1085–1093. doi:10.1007/s10853-011-5899-4
Acknowledgements
The authors are grateful to MEC for financial support for this study under project MAT2009-07811. The DeutchesElektronen-Synchrotron DESY is acknowledged for the provision of beamtime at the P07 beamline of the Petra III synchrotron facility in the framework of proposal I-20120285 EC. The authors would like to thank Dr. A. Rhys Williams for proof-reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcés, G., Requena, G., Tolnai, D. et al. Influence of rare-earth addition on the long-period stacking ordered phase in cast Mg–Y–Zn alloys. J Mater Sci 49, 2714–2722 (2014). https://doi.org/10.1007/s10853-013-7967-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7967-4