Abstract
Novel superabsorbent chitin/carboxymethylcellulose (CMC) hydrogels were successfully prepared from mixture of CMC and chitin solution dissolved in 8 wt% NaOH/4 wt% urea aqueous system at low temperature by crosslinking with epichlorohydrin. The morphology and structure of the resultant composite hydrogels were investigated by scanning electron microscope, thermogravimetry, and Fourier transform infrared spectroscopy. The results indicated that the stiff chains of chitin are as a strong backbone in the hydrogel to support the pore wall, whereas the CMC contributed to water absorption. The maximum swelling ratio in water reached an exciting level of 1300 as the hydrogels still kept an intact appearance. Moreover, the hydrogels exhibited smart swelling and shrinking behaviors in NaCl and CaCl2 aqueous solution, showing salt-responsive adsorption behaviors in different media. This work provided a “green” pathway to prepare chitin-based superabsorbent hydrogels, which would be potential for the application in the biodegradable water-absorbent material field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, superabsorbent hydrogels have attracted considerable attentions because of their specific advantages, such as excellent hydrophilic properties, high swelling ratio, biocompatibility, and abundance in availability. These hydrogels are three-dimensional cross-linked hydrophilic, linear or branched polymers with the ability to absorb large quantities of water, saline, or physiological solutions compared with general absorbing materials [1–3]. Given all these advantages, superabsorbent polymers are widely applied in various fields, such as agriculture, hygienic products, wastewater treatment, drug delivery, tissue engineering, biosensors, and sorbents for the removal of heavy metals and coal dewatering [4–12]. Usually, most of these superabsorbents are prepared from synthetic polymers such as acrylic acid (AA) or acrylamide (AM), which are costly, hardly degradable, and environmentally unfriendly [13–16]. Given the gradual depletion of petroleum resources and the growing environmental pollution crisis from polymer syntheses, natural polymers has become the focus of current studies [15, 17–21]. Recently, cellulose-based and chitosan-based superabsorbent hydrogels prepared by chemical crosslinking have been investigated. In our laboratory, a cross-linked carboxymethylcellulose/cellulose hydrogel, which exhibited superabsorbent capacity and high equilibrium swelling ratio has been synthesized [3]. A superabsorbent polymer from chitosan was provided by Bidgoli et al. [22] via carboxymethylation of chitosan, followed by crosslinking with glutaraldehyde. Chitin is the second most abundant organic material in nature, existing in exoskeletons of crab and shrimp, and it has been first identified in 1884 [23, 24]. However, the chitin-based superabsorbent hydrogels directly prepared from chitin solution have been rarely reported. Chitin and sodium carboxymethylcellulose (CMC) are biocompatible and biodegradable, so they will be widely used in the biomedical field. Chitin is highly crystalline with strong hydrogen bonding, and is very difficult to dissolve in common solvents, only some new solvents such as LiCl/dimethylacetamide (DMAc) mixtures and ionic liquids have been developed to dissolve chitin and to prepare materials [25–28]. Recently, we have successfully developed NaOH/urea aqueous solution as a solvent for chitin via a freezing/thawing method [15, 29–31]. Moreover, pure chitin hydrogel has been successfully prepared, showing excellent physical and chemical properties, providing us a novel pathway for chitin-based material fabrication as superabsorbent [15].
In present work, we attempted to prepare chitin/CMC materials with water-absorbent function by using CMC and chitin solution dissolved in NaOH/urea solvent system. The structure and properties of the chitin/CMC hydrogel were investigated and discussed. The swelling properties and salt sensitivities were measured using the equilibrium swelling ratios (ESR) and the swelling kinetics in different solutions.
Experimental
Materials
Chitin was supplied by Zhejiang Golden-Shell Biochemical Co., Ltd., China. The weight-average molecular weight (M w) of chitin was measured by dynamic light scattering (DLS, ALV/CGS-8F, ALV, Germany) in 5 % LiCl/DMAc (w/w) to be 5.0 × 105 [15]. Its degree of acetylation (DA) was calculated to be 95 % from the nitrogen content according to DA = 1−[(W C/W N − 5.14)/1.72] × 100 %, where W C/W N is the ratio of carbon to nitrogen. Sodium CMC (2.43 × 104) was analytical-grade reagent purchased from Shanghai Chemical Agents Co. Ltd. The degree of carboxymethyl substitution (DS) is 0.7, which is the number of substituents per sugar ring. Epichlorohydrin (ECH) (1.18 g/ml) was of analytical grade, and was used without further purification. All the chemical agents were of analytical grade and were purchased from commercial sources in China.
Preparation of chitin/CMC hydrogels
To prepare the chitin solutions, 3 g chitin was immersed in 97 g of 8 wt% NaOH/4 wt% urea/88 wt% water and stored under refrigeration (−20 °C) for 8 h, then resultant frozen solid was thawed and stirred extensively at room temperature. The freezing/thawing cycle was repeated three times to obtain a transparent chitin solution, with polymer concentration of 3 %. CMC was dissolved in the same solvent to obtain a 3 wt% polymer concentration. The chitin and CMC solutions were mixed with ratio of 5:5, 4:6, 3:7, and 2:8 by weight, respectively. Subsequently, ECH as cross-linker was added to mixture, stirred at 20 °C for 0.5 h to obtain a homogeneous solution, and then kept at 60 °C for 20 min to obtain chitin/CMC hydrogels. Finally, the hydrogels were immersed in distilled water to remove NaOH and urea. Chitin/CMC hydrogels coded as GEL55, GEL46, GEL37, and GEL28 according to the ratio of chitin to CMC of 5:5, 4:6, 3:7, and 2:8 are shown in Table 1.
Characterization
The hydrogel samples were ground into small particles and dried in vacuum at 50 °C for 24 h. The dried samples were analyzed in KBr disks by FTIR (Perkin Elmer Spectrum one, Wellesley, MA, USA). Thermogravimetric analysis (TGA) was carried out on a Pyris TGA linked to a Pyris diamond TA Lab System (Perkin-Elmer Co., USA) at a heating rate of 10 °C min−1 from 40 to 500 °C under nitrogen atmospheres. The surface and fracture section of the hydrogels were observed using a scanning electron microscope (SEM, Hitachi, S-570, Japan). The hydrogels swelled to equilibrium in distilled water at 37 °C for 24 h were frozen directly in liquid nitrogen, immediately snapped, and then freeze-dried under vacuum. The cross section of the hydrogel was coated with carbon and gold, to be observed and photographed.
Swelling measurements
The ESR of hydrogels were investigated in distilled water and various physiological fluids (d-glucose solution: 50 g d-glucose + 1000 mL distilled water; urea solution: 50 g urea + 1000 mL distilled water; physiological saline water: 9 g NaCl + 1000 mL distilled water; and synthetic urine: 8 g NaCl + 1 g MgSO4 + 20 g urea + 0.6 g CaCl2 + 1000 mL distilled water) as well as NaCl and CaCl2 solutions with different concentrations. The ESR value was calculated as
where W s is the weight of the wet hydrogel at swelling equilibrium at 37 °C and W d is the weight of the hydrogel in the dry state. Water uptake (WU) is important for smart hydrogels to characterize the reswelling kinetics of different hydrogel samples after drying. To measure WU, the dried gels were immerged again into distilled water at 37 °C. At each time intervals, the hydrogels were taken out and weighted after removing the excess solution on the surface. The WU value was calculated as
where W t is the weight of wet hydrogel at time t at 37 °C, W d and W s are same as Eq. (1).
Results and discussion
Appearance and structure of the chitin/CMC hydrogels
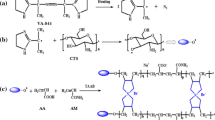
ECH has been widely used for the cross-link of carbohydrates in polysaccharide chemistry [32, 33]. The scheme for cross-link reaction of ECH with chitin and CMC in NaOH/urea solution is shown in Fig. 1. The hydroxyl groups of the chitin were cross-linked with hydroxyl groups of the CMC through nucleophilic attack of the alcoholate anion to form monoethers of chloropropanediols and a new epoxide by chloride displacement. Subsequently, a reaction between the new epoxide and another alcoholate anion occurred, leading to the completion of the cross-link. Reaction conditions and the chemical composition of the chitin/CMC hydrogels are listed in Table 1. The hydrogel samples of GEL55, GEL46, GEL37, and GEL28 having different ratios of chitin to CMC were prepared successfully with ECH as a cross-linker. Figure 2 shows the appearances of GEL37 at different states. The original hydrogel (a) was relatively small; the swollen hydrogel (b), which was immersed and swollen in distilled water, was very transparent and the largest one of these four hydrogels; dried hydrogel (c) had large shrinkage to become very small size; and the swollen hydrogel, which was swollen in 0.1 M NaCl solution, reached a new swelling equilibrium because part of water was extruded the hydrogel (d) shrank obviously. Clearly, the chitin/CMC hydrogels with high water absorbability were successfully constructed from natural polymers, which are biodegradable. We did not prepared pure CMC hydrogel, because it cannot hold lots of water to maintain the stable appearance at the same time. This indicated that the relative stiff chitin chains acted as a strong backbone to support the pore wall [15].
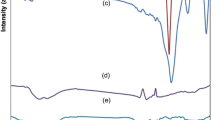
The FTIR spectra of the chitin and chitin/CMC hydrogels are shown in Fig. 3. Usually, IR spectrum of α-chitin exhibited major peaks at 3446 cm−1 for –OH stretching vibration, 3260 cm−1 for –NH stretching vibration, 1660 cm−1 (amide I), 1623 and 1557 cm−1 (amide II) [34]. Compared with the chitin/CMC hydrogels, the peaks at 3446 cm−1 for stretching vibration of –OH groups disappeared exactly in the spectrum of chitin/CMC hydrogels. The new peaks at 1600 and 1420 cm−1 in the chitin/CMC hydrogels can be attributed to –COOH stretching and bending, respectively [35]. This can be explained that the –COOH groups of CMC existed in the hydrogels, and the crosslinking between chitin and CMC succeeded.
SEM images of the cross section of the chitin/CMC hydrogels are shown in Fig. 4. The results displayed well-defined, interconnected, three-dimensional porous structures with significant micropores. These pores acted as water permeation regions, where water easily diffused into. Due to the electrostatic repulsions caused by the carboxylate anions (COO−) in CMC, the space in the networks of hydrogels increased. Thus, the size of pores increased with an increase of CMC contents, leading to a more open and loose structure. In view of these results, chitin acted as a backbone in the hydrogel to strengthen the porous architectures, whereas the CMC contributed to control the size of pore. Therefore, large number of water molecules could be easily absorbed and diffused into hydrogels to form large pores, leading to higher swelling ratio.
Figure 5 shows the TG and DTG curves of CMC, chitin, chitin/CMC mixture, and GEL55. The weight loss around 330 °C of chitin was attributed to the decomposition of chitin, and the peak at 290 °C resulted from the decomposition of CMC. Moreover, for the chitin/CMC mixture, there were two peaks in the DTG, one is at 290 °C, whereas another is at 330 °C. For the GEL55, the peaks at 330 °C in the chitin/CMC mixture disappeared, and only one peak at 290 °C was displayed in the DTG. This indicated that only one kind of homogeneous substance existed in GEL55, as a result of the crosslinking reaction.
Swelling properties of the hydrogels
Figure 6 displays the influence of the CMC content on the swelling ratio of the chitin/CMC hydrogels in distilled water at 37 °C. Generally, superabsorbent can absorb hundreds of times its own weight of water. All the samples exhibited high equilibrium swelling ratio, indicating that the chitin/CMC hydrogels were superabsorbent hydrogels. The equilibrium swelling ratio of the chitin/CMC hydrogels increased rapidly with an increasing of the CMC contents. This confirmed further that highly hydrophilic carboxyl group of CMC could absorb a lot of water to fill up pores, leading to the large space. Additionally, because of heating during crosslinking reaction, physical crosslinking occurred in the hydrogels, and chitin/NaOH/urea aqueous solution underwent irreversible gelation process [15, 36]. It was noted that the equilibrium swelling ratio decreased with an increase of chitin content. This could be explained that the chitin chains can be self-entangled easily by hydrogen bonds in high chitin concentration solution, leading to the reducing of the space. The maximum swelling ratio of the hydrogels was more than 1200, which was absolutely higher than that prepared from chitin or cellulose derivative [37, 38]. High swelling ratio is very important for biomaterials to apply widely in the biomedical and food fields.
To investigate the effects of simulated biological fluids on the swelling phenomena of the chitin/CMC hydrogels and evaluate their suitability as biomaterials, we studied their swelling ratios in four simulated biological solutions. Figure 7 shows the swelling ratios of the chitin/CMC hydrogels. The size of hydrogels were appreciably reduced in the d-glucose, urea, physical saline water, and synthetic urine solutions when compared with the data measured in distilled water. All the hydrogels exhibited the same shrinking behaviors in a given solution, because the electrostatic repulsion effects caused by the charges of the carboxyl groups on the hydrogel backbones were inhibited. The swelling ratio of hydrogels in d-glucose solution was as high as in distilled water, whereas the swelling ratios decreased quickly in physical saline water and in synthetic urine. This could be explained that the charge screening effect caused by cations (Na+, K+, Mg2+, and Ca2+) in physical saline water and synthetic urine, which could reduce the anion–anion electrostatic repulsions, leading to a decrease of the osmotic pressure between the biopolymer network and the external solution [39]. According to the Donnan osmotic pressure equilibrium, the more the movable counterions in a solution, the lower the osmotic pressure inside the hydrogel, then the hydrogel shrank [40]. The order of the swelling ratio of the chitin/CMC hydrogels in different biological fluids was as follows: d-glucose solution > urea solution > physical saline water ≈ synthetic urine. It is attributed to NaCl, which is the main component to make an important contribution to the swelling of hydrogels [41].
Figures 8 and 9 display the effect of salt concentration on the swelling ratio of the chitin/CMC hydrogels. In NaCl solution, the swelling ratio of the hydrogels decreased with the increasing of the ionic strength of the solution. The hydrogels with higher CMC contents exhibited more significant decline of swelling ratio with the increase of the NaCl concentration. In CaCl2 aqueous solution, compared with NaCl, the cationic charge of CaCl2 is much higher, thus the swelling ratio decreased quickly, in accord with the Donnan osmotic pressure equilibrium. Since the distinction in the concentration of mobile ions between the hydrogel and external solution was reduced, the osmotic swelling pressure of mobile ions within the hydrogel decreased, resulting in shrinkage of the hydrogel [40].
The reswelling behaviors of the dried chitin/CMC hydrogels in distilled water at 37 °C are shown in Fig. 10. The reswelling capabilities of the hydrogels increased with the increasing of CMC content. The water uptake of dried GEL28 reached 98 %, and dried GEL55 exhibited a low value of 72 %. It probably due to the strong hydrogen bonding interactions between the hydroxyl groups of chitin and carboxyl groups of CMC that occurred during the drying process, and it extremely reduced the relaxation and expansion of molecular chains. Moreover, the greater number of negative charges COO− of CMC in the gels magnified the molecular chains repulsion, leading to the enlarging of meshes and promoting of the water uptake. This indicated that it was very easy for the higher CMC content samples to reach their initial swollen state. The results further proved that the water uptakes of the hydrogels increased with an increase of CMC content in the hydrogels.
Conclusions
The superabsorbent hydrogels were successfully prepared by crosslinking chitin and CMC in NaOH/urea aqueous solution with epichlorohydrin. The chitin/CMC hydrogels exhibited homogeneous porous structure and large surface area. The stiff chitin chains contributed to support the pore wall, whereas the highly hydrophilic CMC acted as adsorbent of water, leading to the high swelling ratio. The chitin/CMC hydrogels exhibited superabsorbent capacity and high equilibrium swelling ratio, depending on the amount of CMC. The hydrogels were sensitive to inorganic salts aqueous solution, physical saline water, and synthetic urine, showing smart swelling and shrinking behaviors. This would be very important in the biomaterial fields.
References
Pourjavadi A, Harzandi A, Hosseinzadeh H (2004) Modified carrageenan 3. Synthesis of a novel polysaccharide-based superabsorbent hydrogel via graft copolymerization of acrylic acid onto kappa-carrageenan in air. Eur Polym J 40:1363
Pourjavadi A, Harzandi A, Amini-Fazl M (2008) Taguchi optimized synthesis of collagen-g-poly(acrylic acid)/kaolin composite superabsorbent hydrogel. Eur Polym J 44:1209
Chang C, Duan B, Cai J, Zhang L (2010) Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur Polym J 46:92
Murthy P, Mohan Y, Varaprasad K, Sreedhar B, Raju K (2008) First successful design of semi-IPN hydrog–silver nanocomposites: a facile approach for antibacterial application. J Colloid Interface Sci 318:217
Kim J, Lee K, Hefferan T, Currier B, Yaszemski M, Lu L (2008) Synthesis and evaluation of novel biodegradable hydrogels based on poly(ethylene glycol) and sebacic acid as tissue engineering scaffolds. Biomacromolecules 9:149
Adhikari B, Majumdar S (2004) Polymers in sensor applications. Prog Polym Sci 29:699
Pourjavadi A, Ghasemzadeh H, Soleyman R (2007) Synthesis, characterization, and swelling behavior of alginate-g-poly(sodium acrylate)/kaolin superabsorbent hydrogel composites. J Appl Polym Sci 105:2631
Chu M, Zhu S, Li H, Huang Z, Li S (2006) Synthesis of poly(acrylic acid)/sodium humate superabsorbent composite for agricultural use. J Appl Polym Sci 102:5137
Kamat M, Malkani R (2003) Disposable diapers: a hygienic alternative. Indian J Pediatr 70:879
Yi J, Zhang L (2008) Removal of methylene blue dye from aqueous solution by adsorption onto sodium humate/polyacrylamide/clay hybrid hydrogels. Bioresour Technol 99:2182
Sadeghi M, Hosseinzadeh H (2008) Synthesis of starch-poly(sodium acrylate-co-acrylamide) superabsorbent hydrogel with salt and pH-responsiveness properties as a drug delivery system. J Bioactive Compat Polym 23:381
Ende M, Hariharan D, Peppas N (1995) Factors influencing drug and protein transport and release from ionic hydrogels. React Funct Polym 25:127
Wang J, Wang W, Wang A (2010) Synthesis, characterization and swelling behaviors of hydroxyethyl cellulose-g-poly(acrylic acid)/attapulgite superabsorbent composite. Polym Eng Sci 50:1019
Narimani F, Zohuriaan-Mehr MJ, Kabiri K, Bouhendi H, Omidian H, Najafi V (2012) Overentrant swelling behaviour of poly(potassium, 3-sulfopropyl acrylate-acrylic acid) gels. J Polym Res 19:7
Chang C, Chen S, Zhang L (2011) Novel hydrogels prepared via direct dissolution of chitin at low temperature: structure and biocompatibility. J Mater Chem 21:3865
Wu F, Zhang Y, Liu L, Yao J (2012) Synthesis and characterization of a novel cellulose-g-poly(acrylic acid-co-acrylamide) superabsorbent composite based on flax yarn waste. Carbohydr Polym 87:2519
Tang H, Zhou W, Lu A, Zhang L (2013) Characterization of new sorbent constructed from Fe3O4/chitin magnetic beads for the dynamic adsorption of Cd2+ ions. J Mater Sci. doi:10.1007/s10853-013-7684-z
Zohuriaan-Mehr M, Omidian H, Doroudiani S, Kabiri K (2010) Advances in non-hygienic applications of superabsorbent hydrogel materials. J Mater Sci 45:5711. doi:10.1007/s10853-010-4780-1
Hua F (2001) Synthesis of self-crosslinking sodium polyacrylate hydrogel and water-absorbing mechanism. J Mater Sci 36:731. doi:10.1023/A:1004849210718
Liu H, Wang C, Gao Q, Tong Z (2008) Fabrication of novel core-shell hybrid alginate hydrogel beads. Int J Pharm 351:104
Dond H, Xu Q, Li Y, Mo S (2008) The synthesis of biodegradable graft copolymer cellulose-graft-poly(l-lactide) and the study of its controlled drug release. Colloid Surf B 66:26
Bidgoli H, Zamani A, Taherzadeh M (2010) Effect of carboxymethylation conditions on the water-binding capacity of chitosan-based superabsorbents. Carbohydr Res 345:2683
Vrieze S, Westbroek P, Camp T, Langenhove L (2007) Electrospinning of chitosan nanofibrous structures: feasibility study. J Mater Sci 42:8029. doi:10.1007/s10853-006-1485-6
Zhang L, Tang H, Chang C (2010) China Patent ZL, 201010201892.3
Rosa BA, Quintana P, Ardisson P, Limón JM, Gil JJ (2012) Effects of thermal treatments on the structure of two black coral species chitinous exoskeleton. J Mater Sci 47:990. doi:10.1007/s10853-011-5878-9
Ghosh A, Ali M (2012) Studies on physicochemical characteristics of chitosan derivatives with dicarboxylic acids. J Mater Sci 47:1196. doi:10.1007/s10853-011-5885-x
Tian F, Liu Y, Hu K, Zhao B (2003) The depolymerization mechanism of chitosan by hydrogen peroxide. J Mater Sci 38:4709. doi:10.1023/A:1027466716950
Chang C, Peng J, Zhang L, Pang D (2009) Strongly fluorescent hydrogels with quantum dots embedded in cellulose matrices. J Mater Chem 19:7771
Tang H, Zhang L, Hu L, Zhang L (2013) Application of chitin hydrogels for seed germination, seedling growth of rapeseed. J Plant Growth Regul. doi:10.1007/s00344-013-9361-5
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209–210:218
Tang H, Chang C, Zhang L (2011) Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway. Chem Eng J 173:689
Miguel I, Rieumajou D, Betbeder D (1999) New methods to determine the extent of reaction of epichlorohydrin with maltodextrins. Carbohydr Res 319:17
Chang C, Lue A, Zhang L (2008) Effects of crosslinking methods on structure and properties of cellulose/PVA hydrogels. Macromol Chem Phys 209:1266
Dong J, Ozaki Y (1997) FTIR and FT-Raman studies of partially miscible poly(methyl methacrylate)/poly(4-vinylphenol) blends in solid states. Macromolecules 30:286
Tang H, Lu A, Li L, Zhow W, Xie Z, Zhang L (2013) Highly antibacterial materials constructed from silver molybdate nanoparticles immobilized in chitin matrix. Chem Eng J 234:124
Cai J, Zhang L (2006) Unique gelation behavior of cellulose in NaOH/urea aqueous solution. Biomacromolecules 7:183
Liu T, Qian L, Li B, Li J, Zhu K, Deng H, Yang X, Wang X (2013) Homogeneous synthesis of chitin-based acrylate superabsorbents in NaOH/urea solution. Carbohydr Polym 94:261
Yoshimura T, Matsuo K, Fujioka R (2006) Novel biodegradable superabsorbent hydrogels derived from cotton cellulose and succinic anhydride: synthesis and characterization. J Appl Polym Sci 99:3251
Zhao Y, Kang J, Tan T (2006) Salt-, pH- and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly(aspartic acid) and poly(acrylic acid). Polymer 47:7702
Barbucci R, Magnani A, Consumi M (2000) Swelling behavior of carboxymethylcellulose hydrogels in relation to cross-linking, pH, and charge density. Macromolecules 33:7475
Kim S, Shin S, Shin D, Kim I, Kim S (2005) Synthesis and characteristics of semi-interpenetrating polymer network hydrogels based on chitosan and poly(hydroxy ethyl methacrylate). J Appl Polym Sci 96:86
Acknowledgements
This work was supported by National Basic Research Program of China (973 Program, 2010CB732203), the Major Program of National Natural Science Foundation of China (21334005), and the National Natural Science Foundation of China (20874079 and 51203122).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, H., Chen, H., Duan, B. et al. Swelling behaviors of superabsorbent chitin/carboxymethylcellulose hydrogels. J Mater Sci 49, 2235–2242 (2014). https://doi.org/10.1007/s10853-013-7918-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7918-0