Abstract
We report on the homogeneity, structural and luminescence properties of ceria nanoparticles doped with Eu3+. Eu3+ in concentration of 1, 5 and 10 wt% was incorporated via wetness impregnation into preformed ceria nanoparticles followed by calcination in air at 1000 °C. A remarkable homogeneity of Eu3+-ceria solid solutions is measured for ceria grown by citrate and micro-emulsion methods using Raman, Diffuse Reflectance in UV–Vis, photoluminescence spectroscopies and X-ray diffraction, even for the Eu3+ concentration of 10 wt%. The emission properties of all Eu3+-doped ceria samples are well-characterized by a two main centre model assigned to perturbed and isolated Eu3+ centres. These centres correspond to Eu3+ located in the nearest (local symmetry lower than cubic and Eu3+-oxygen coordination lower than eight) and next-nearest-neighbour positions (cubic local symmetry and eightfold Eu3+-oxygen coordination) to oxygen vacancy, respectively. With increase of Eu3+ concentration, both the oxygen vacancy concentration and the relative contribution of the perturbed Eu3+ centre to the total emission increase. It is established that the characteristic emission and excitation spectra of the two main Eu3+ centres as well as the overall multisite distribution of Eu3+ within ceria lattice are intrinsic properties of Eu3+-doped ceria since these do not depend on synthesis route, nanoparticle size and Eu3+ concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ceria (CeO2) doped with trivalent yttrium and lanthanide (Ln3+) ions is extensively investigated due to their elevated oxygen storage capacity (OSC) combined with the ability to shift easily between the redox pair Ce4+ and Ce3+ oxidation states [1]. Besides its classical use as a catalyst or catalyst support, ceria also presents interest for biomedicine, radical scavenging antioxidants and biological labels applications [2, 3]. The ‘marriage’ of the europium dopant and ceria host is expected to enhance these applications due to the luminescence properties of Eu3+ and also to shed light on the local structure properties due to the well-known luminescence sensitivity of Eu3+ to nearest ligand (oxygen) environments [4, 5]. To date, literature on Eu3+ doped ceria evidences for considerable changes of host and dopant physical properties as a result of the symmetry diversity (cubic to lower symmetries) at Eu3+ sites, Eu3+ concentration, thermal treatment conditions, Ce3+ concentration and so on [2, 6–15]. It is generally considered that the lanthanide dopants with larger ionic radii than Gd3+ such as Eu3+ repel the oxygen vacancy at the next-nearest-neighbour (NNN) location [1], and thus the Eu3+ may preserve the eightfold coordination of Oh symmetry which characterizes the lattice Ce4+ sites. However, literature offers strong spectroscopic evidences that besides cubic sites, Eu3+ is distributed also on a second site with a non-centrosymmetric, lower symmetry, and its relative contribution to the total emission is increased with concentration or/and calcination temperature. Concerning the nature of this second site, this is not fully elucidated in literature cited above, being attributed to one of the following factors: conversion of ‘bulk’ to surface Eu3+, interstitial sites, Eu3+-substituted Ce4+ associated with defect such as oxygen vacancy or increased covalency of the Eu3+-oxygen bond or combined effect of the excess of oxygen vacancies, increased Ce3+/Ce4+ ratio and phase miscibility due to reduction process. Furthermore, issues such as the dependence of the local structure and distribution of Eu3+ on ceria lattice sites on the synthesis method, size and concentration are not addressed yet.
Here, we report on homogeneity, structural and luminescence properties of Eu3+ doped CeO2 nanoparticles presented using X- ray Diffraction (XRD), Diffuse Reflectance in UV–Vis (DR–UV/Vis), Raman and photoluminescence spectroscopies. Ceria nanoparticles were synthesised by micro-emulsion and citrate methods or purchased from Aldrich. Eu3+ was added, via wetness impregnation, on preformed ceria nanoparticles in concentration of 1, 5 and 10 wt%. The homogeneity of the Eu3+-CeO2 solid solutions was carefully established using all mentioned above techniques. Next, the characteristic emission and excitation spectra of the main Eu3+ centres, their nature and dependencies on the synthesis method, nanoparticle size and Eu3+ concentration were determined. To our knowledge, this is the first study aiming at establishing the intrinsic distribution of Eu3+ on ceria lattice sites.
Materials and methods
Synthesis
The ceria nanocrystals were obtained using a recently developed micro-emulsion reaction method under soft conditions (ow) [16], citrate method (cit) [17] and commercial (com, Aldrich, 99.995 % trace metals basis; it was used as received). In our preparation procedure, Ce(NO3)3·6H2O was dissolved in 100 mL of distilled water heated at 60 °C and the solution was stirred for 10 min. Citric acid (Merck 244) was added with 10 wt% excess over the stoichiometric quantity for the complete complexation of the metal ions to this solution and the stirring was continued for 1 h at 60 °C. The solution was partially dehydrated in a rotary evaporator at 40 °C until the appearance of a colourless gel. The gel was then dried for 5 h under vacuum at 60 °C and then at 120 °C in air for another 12 h. During this treatment an intense production of nitrogen oxide vapours occurred. A spongy yellowish amorphous solid was obtained.
For the ow preparation, the micro-emulsion system was: water/Synperonic®10/6 (hexaethylene glycol isodecyl ether, technical grade, Croda)/hexane. Cerium (III) 2-ethylhexanoate (Aldrich) was used as organometallic precursor. The composition used was: 64.5 wt% water, 21.5 wt% surfactant and 14 wt% oil phase (hexane solution of Cerium (III) 2-ethylhexanoate). All the components were mixed to obtain an isotropic solution (micro-emulsion) at a temperature of 35 °C. The pH of the solution was then increased to 11 by the addition of concentrated ammonia (25 %, Merk) under vigorous stirring and left for 48 h. The obtained ceria powder was washed using a mixture of ethanol and chloroform (1:1), dried overnight at 70 °C and grinded using an agate pestle and mortar. For the preparation of Eu3+ containing samples (1, 5 and 10 wt%), 1 g of dried CeO2 was impregnated with the required amount of a 0.004 M solution of EuCl3·6H2O (Fluka). The suspension was stirred 12 h at 60 °C and then water was evaporated at 80 °C. Drying of the samples was continued at 80 °C in vacuum for 4 h. The samples were calcined in air for 4 h at 1000 °C with a heating rate of 5 °C/min.The samples were denoted as CE(ow)-x, CE(cit)-x and CE(com)-x where x refers to Eu3+ concentration (1, 5 and 10 wt%).
Characterization
Powder X-ray diffraction (XRD) patterns were recorded on a Schimadzu XRD-7000 diffractometer using Cu Kα radiation (λ = 1.5418 Å, 40 kV, 40 mA) at a step of 0.2 degrees and a scanning speed of 2 degrees min−1 in the 10°–90° 2Θ range. Crystalline phase was identified by comparasion of the XRD patterns with the JCPDS database. Diffuse reflectance UV–Vis spectra were recorded at room temperature on a Analytik Jena Specord 250 spectrophotometer with an integrating sphere for reflectance measurements and Spectralon as the reflectance standard. DR–UV/Vis spectra of the catalysts were recorded in reflectance units and were transformed in Kubelka–Munk remission function F(R). The differential thermal analysis and thermogravimetric analysis were carried out using a TG–DTA analyser (Shimadzu DTG-60 Simultaneous DTA-TG Apparatus) in nitrogen atmosphere under the heating rate of 10 °C/min from room temperature to 900 °C and using alumina as reference. Raman analysis was carried out with a Horiba Jobin–Yvon–Labram HR UV–Visible–NIR Raman Microscope Spectrometer, at a laser wavelength of 514 and 488 nm, respectively.
Photoluminescence measurements
The photoluminescence (PL) measurements were carried out using a Fluoromax 4P spectrofluorometer (Horiba) operated in both the fluorescence and the phosphorescence mode. The repetition rate of the xenon flash lamp was 25 Hz, the integration window varied between 300 ms and 3 s, the delay after flash varied between 0.03 and 10 ms, and up to 100 flashes were accumulated per data point. The slits were varied from 0.001 to 10 nm in excitation as well as emission. PL decays were measured using the ‘decay by delay’ feature of the phosphorescence mode. Time-resolved emission spectra (TRES) were recorded at room temperature using a wavelength tunable NT340 Series EKSPLA OPO (Optical Parametric Oscillator) for samples excitation at 340–360 nm, 457, 488, 460–470 and 514 nm operated at 10 Hz as excitation light source and an intensified CCD camera (Andor Technology) coupled to a spectrograph (Shamrock 303i, Andor) as detection system. The TRES were collected using the box car technique. The initial gate delay (delay after laser pulse, δt) was set to 1 μs and the gate width δt was varied between 10 and 300 μs. The number of accumulations per laser pulse was varied from 50 up to 500. The PL was detected in the spectral range of 500 nm < λ em < 750 nm with a spectral resolution of ~0.25 nm. All measurements were performed at room temperature (300 K). The PL decays were analysed by fitting with a multiexponential function f(t) using the commercial software (OriginPro 8):
where A i is the decay amplitude, B is a constant (the baseline offset) and τ i is the time constant of the decay i. The average PL lifetime was calculated as amplitude-weighted
Results and discussion
Structural and homogeneity properties
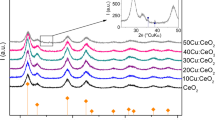
Figure 1 presents the X-ray diffractograms of investigated CE samples. In all cases, the diffraction peaks can be indexed as a pure cubic fluorite CeO2 structure, which coincide well with the standard data of CeO2 (PDF 00-043-1002). Except for commercial ceria, for which a strong asymmetry was observed around 220 reflection at ~47.5°, no other phases were observed even for the greatest Eu3+ concentration of 10 wt%. With increase of Eu3+ amount from 1 to 10 wt%, the crystallite size decreased from 41 to 30 nm in CE(cit) and from 38 to 30 nm in CE(ow) as determined with Debye–Scherrer equation. No significant changes of the particle size were observed for CE(com) samples, with crystallite size varying only slightly from 52 to 55 nm for the similar concentration range of Eu3+. This is most probably due to the thermal pretreatment done by the manufacturer, which prohibits the full incorporation of Eu3+ dopants (see also thermo-gravimetric analysis of the undoped ceria samples in Supplemental Information).With the increase of Eu3+ concentration, the peak positions of reflections shift to lower 2θ angles which indicates that the doped ceria lattice expands with the increase in dopant content. This result is a consequence of the greater ionic radius of Eu3+ compared to Ce4+, in eightfold coordination (1.066 and 0.97 Å), respectively [18].
Overall, the XRD patterns sustain an effective incorporation of Eu3+ in the network of ceria for CE(cit) and CE(ow) nanoparticles. The capability of ceria to dissolve lanthanide oxides (in concentration of up to 30 % and using a milder calcinations, of 400 °C) was recently observed by Corma et al. [19]. The property is assigned to nanometric dimensions of ceria since for the micrometric ceria, a similar homogenous distribution of the lanthanide dopant within the lattice was not found.
DR-UV/Vis spectra (Fig. 2) show a strong absorption band below 400 nm which is typical for ceria, assigned to the charge-transfer transition from O2− (2p) to Ce4+ (4f) orbitals in CeO2 [20]. It is noteworthy that the Eu3+ f–f absorptions are not detected except for the case of commercial ceria for which peaks at 466 and 535 nm are assignable to Eu 7F0–5D2, 1 absorptions in the Eu2O3 oxide.
Next, the formation of homogenous solid solutions and defects is described using Raman spectroscopy. To overcome possible ambiguities in the structural information obtained from Raman spectra, two Vis laser excitation wavelengths at 488 and 514 nm were employed. As recently shown, luminescence bands of lanthanides usually detected outside the phonon range (>800 cm−1) can interfere with phonon bands of ceria based materials, [21] even when present as unintentional impurities (concentration in the ppm range). On the other side, the presence of the luminescent impurities can play a positive role when used as structural probes. Therefore, we analyse the vibrational properties of the ceria host and luminescence of Eu3+ from the Raman spectra measured within extended spectral region, namely from 150 to 4000 cm−1, [22, 23].
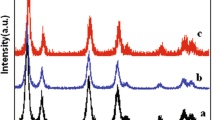
Figure 3 and Fig. S2 gathers the Raman spectra of Eu3+ doped CeO2 (ow), CeO2 (com) and CeO2 (cit), respectively. Irrespective of the sample, the Raman spectra are characterized in the spectral range of phonon bands, by a strong, narrow phonon band around 460–470 cm−1 which corresponds to the F2g mode of the CeO2 fluorite phase along with weaker bands around 250, 600 and 1165 cm−1, due to second-order transverse acoustic (2TA) defect-induced (D) and second-order longitudinal optical (2LO) modes, respectively [24].
Further, the enhancement of the spectral feature around 550 cm−1 together with the F2g band shift by ~4 cm−1 to lower energy is evident for the concentrated samples (ow, cit). The spectral feature at 550 cm−1 is assigned to the oxygen vacancies [25] which are generated upon Ce4+ substitution by Eu3+. With the increasing Eu3+ concentration, the F2g mode becomes asymmetric, broader and slightly shifted to lower wavenumber which is related to the combined effects of strain and phonon confinement resulted from the decrease of the crystallite size [26, 27]. These observations agree with the XRD results since the crystallite size decreases when increasing the Eu3+ content (Fig. 1). Comparative analysis of the Raman spectra of CE(ow) and CE(cit) samples (Fig. S2) measured at 488 and 514 nm did not evidence bands assignable to phonon modes of Eu2O3 (such as a feature at 610 cm−1) according to Hernandez et al. [27]. For CE(com) no difference between CE(com)-1 and CE(com)-10 was measured which agrees with limited incorporation of Eu3+ in ceria lattice determined previously by XRD and DR–UV/Vis analysis. Instead, very weak bands around 340 cm−1 observed at the two excitation wavelengths may be tentatively assigned to the strongest phonon mode (Fg) of Eu2O3 cubic phase [28]. Besides the phonon bands of CeO2, several narrow and relatively intense bands in the spectral range of ~1500–4000 cm−1 are observed which change strongly with concentration and excitation wavelength. These lines correspond to the electronic luminescence transitions of Eu3+ substituting for Ce4+ sites in CeO2 lattice and their detailed description is presented below. It is noteworthy to mention here that the luminescence of Eu3+ in Eu2O3 parasite forms is absent in the Raman spectra, since Eu2O3 displays strongly different shape and peaks values [29] as those illustrated in Fig. 3 (see also Fig. S2).
Intrinsic structure of Eu3+ centres in ceria nanoparticles
Figure 4 illustrates the steady-state emission and excitation spectra of CE(ow)—1, 5, 10 and CE(cit)—10 samples. The emission spectra were excited at two excitation wavelengths, corresponding to the maximum of the charge-transfer absorption band of ceria corresponding to the Ce4+–O2− transition (340–370 nm) and 7F0–5D2 absorption of Eu3+ (around 466 nm). Upon excitation into the CT band, the emission is dominated by the 5D0–7F1 MD transition at 591 nm which corresponds to Eu3+ located in the high symmetry (Oh) sites of ceria lattice. For Oh symmetry, only transitions with ΔJ = 0, 1 are allowed (except for J = 0↔−J = 0). Because ionic radius of Eu3+ ion is higher than that of Ce4+ in eightfold coordination, it is expected that Eu3+ interacts with the oxygen vacancy in the next-nearest-neighbour (NNN) position, and the local symmetry at Eu3+ sites corresponds to that of Ce4+ in cubic fluorite sites [1]. However, the existence of relative strong emission lines in the range of 5D0–7F2 electric dipole (ED) transition (at 611 and 632 nm) and 5D0–7F0 (at 580 nm) sustains for multisite distribution of Eu3+ in ceria. The relative emission contribution of 611 and 632 nm peaks to the overall emission intensity is enhanced with Eu3+ concentration and is changed by shifting the excitation wavelength from ceria CT band to Eu3+ f–f absorption at 466 nm. It is acknowledged that the intensities ratio defined as I (5D0–7F2)/I (5D0–7F1) known as asymmetry ratio, (R) is sensitive to the local asymmetry of the Eu3+-oxygen coordination polyhedron, being higher for low local symmetry at Eu3+ sites [30, 31].
a, b Steady-state excitation and emission spectra of Eu3+ doped ceria (ow) nanoparticles. Excitation, emission wavelengths and values of the asymmetry ratio, R are indicated on the Figure; c Time-resolved excitation spectra measured for CE (cit)-10 corresponding to cubic centre emission (591 nm) measured at two different delays after the lamp pulse
The asymmetry ratio increases with the increase of concentration from 0.45 to 1.28 (excitation into CT band) and from 1.5 to 1.9 (excitation at 466 nm) (Fig. 4a, b). Here, R refers to an ‘average’ local symmetry, as emission spectra are related to a sum of emissions corresponding to cubic (isolated) and low symmetry (perturbed) Eu3+ centres. The perturbation is most probably related to short-range interaction between Eu3+ and oxygen vacancy located in the nearest-neighbour (NN) site which lowers the symmetry, removes the inversion centre, allowing the observance of the ED transitions. The increase with Eu3+ concentration of the relative contribution of the perturbed Eu3+ centre to the total emission parallels the increase of oxygen vacancy concentration as established with Raman spectra (see insets in Figs. 3 and S2).
To establish the sensitization pathway of Eu3+ emission in the two sites, we compare the steady-state and time-resolved excitation spectra corresponding to the cubic centre (emission set at 591 nm) and perturbed centre (emission set at 611 or 632 nm) for the three Eu3+ concentrations. Figure 4a, b illustrates the case for Eu3+-CeO2 (ow) and shows that the cubic centre is excited predominantly via the CT band while the perturbed centre is excited predominantly via the Eu3+ f–f absorptions (with that at 466 being the most intense). We should, however, remark the presence of a non-negligible spectral overlap between the two centre emissions at both wavelengths of 591 and 632 nm. For example, the contribution of the CT band in the excitation of perturbed centre emission might be overestimated since around 632 nm, the emission of the cubic centre, though weak, is non-zero. Conversely, the contribution of the 466 nm absorption in the excitation spectrum of cubic centre emission (at 591 nm) might be also overestimated since around 591 nm, the emission of the perturbed centre is not negligible. Such non-selectivity effects are confirmed by the time-resolved excitation spectra (at 591 nm) measured at two different delays, δt, after the lamp pulse and exemplified in Fig. 4c with CE(cit)-10. At a shorter delay (0.03 ms) both emissions are present whereas imposing a longer delay of 5 ms, only the emission of the longer-lived cubic centre is detected. In the latter case, the excitation spectrum contains basically the CT band absorption followed by much weaker f–f absorptions. Nevertheless, despite the difficulty in spectral separation of the two emissions, we can conclude that the perturbed centre is preferentially excited via—f–f absorption at 466 nm whereas the cubic centre is preferentially sensitized via the charge-transfer absorption band of CeO2.
Figure 5 illustrate the temporal evolution of the (peak normalized) PL spectra of Eu3+-CeO2 (ow; cit) excited into the CT band and at 466 nm and collected at three delays after the laser pulse. Also included in Fig. 5 is the temporal evolution of the asymmetry ratios for delay increasing from few μs up to 17.5 ms. For each case, the longest delay was limited by the signal-to-noise ratio. As a general trend, the temporal evolution sustains the superposition of two main emissions with strongly different shapes and decay times. At short delays, the emissions of both Eu3+ centres are measured. The emission of the low symmetry centre prevails for the concentrated sample (10 wt%) up to 2 ms after the laser pulse for excitation into CT band and up to 5 ms for excitation at 466.6 nm, whereas the emission characteristic of the cubic centre dominates the emission of the diluted sample when excited into the CT band. Such trends agree with the steady-state emission and excitation spectra illustrated in Fig. 4. At long delays, the emission of the perturbed centre is quenched, and the emission corresponds largely to the cubic Eu3+ centre. The ‘true’ emission spectrum of the cubic centre is extracted from the delayed PL spectra measured at delay times which typically exceed 10–20 ms and by use of up to 500 accumulations per laser pulse. It is noteworthy to mention that the obtained delayed emission spectra are highly similar, irrespective of sample type (see also Fig. S3) and Eu3+ concentration.
These findings demonstrate that the local oxygen environments at Eu3+ cubic sites in ceria do not depend on synthesis, size and dopant concentration. Furthermore, supposing that the overall emission is given by these two non-interacting centres, we can approximate the emission spectrum of the low symmetry centre as the difference spectrum between the total and cubic related emission spectra, respectively. The difference spectra were obtained both from steady-state emission spectra measured at different concentrations or from time-resolved PL spectra measured at several pairs of delays. The characteristic emission spectra related to the cubic and perturbed Eu3+ centres are gathered in Fig. 6. The emission spectrum of the cubic centre (determined experimentally from the delayed emission spectra) is dominated by the emission at 591 nm with small peaks in the spectral region of the forbidden ED transitions (600–640 nm) and has an asymmetry ratio of 0.55 ± 0.15. The emission spectrum of the perturbed centre is dominated by relatively strong ED emission at 611 and 632 nm, followed by several lines around 5D0–7F1 and 5D0–7F0 emissions around 591 and 580 nm, respectively. The asymmetry ratio, R, of 3.15 ± 0.25 confirms a low symmetry around Eu3+ centres, which is most probably defined by a short-range interaction between Eu3+ and nearest-neighbour (NN) oxygen vacancies [23]. However, the R value is slightly overestimated since the emission of the perturbed Eu3+ centre at 591 nm is discarded in the difference spectra (effect marked with ‘*’ on Fig. 6).
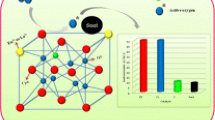
Though the co-existence of two main Eu3+ centres is consistent with the emission data described here (see also Scheme 1), the actual picture of Eu3+ interactions with ceria host is more complex. To demonstrate this, we have performed a close examination of 5D0–7F0 emission. Since both 5D0 and7F0 levels are non-degenerate and cannot be split by the crystal-field, the line shape is informative for the number of different Eu3+ crystal-field environments or centres.
Two centre model in Eu3+ doped ceria nanoparticles. These centres correspond to Eu3+ located in the nearest (local symmetry lower than cubic and Eu3+-oxygen coordination lower than eight) and next-nearest-neighbour positions (cubic local symmetry and eightfold Eu3+-oxygen coordination) to oxygen vacancy, respectively
Figure 7 gathers the lineshape analysis of 5D0–7F0 emission in CE(ow) and CE(cit) nanoparticles by the use of selective laser excitation (λ ex = 464.8, 465.5, 465.7, 465.8, 466.2, 466.6 and 514 nm). With change of the excitation wavelength, the shape, peak position (peaks at 578.44 ± 0.4; 579.08 ± 0.1; 579.56 ± 0.2; 580 ± 0.1 nm) and width of this emission change reflecting the co-existence of minimum four perturbed centres in agreement with the low-temperature data reported earlier [32].
We have also measured PL decays of CE(ow), CE(cit) and CE(com) measured on different emission wavelengths, such as λ em = 580, 591, 611, 632 and 713 nm corresponding to 5D0–7F0, 5D0–7F1, 5D0–7F2, 5D0–7F2 and 5D0–7F4 transitions, respectively, with excitations into CT band and at 466 nm. To reveal the main dependencies of PL decays on concentration, emission or excitation wavelengths, selected curves are represented in Fig. 8. As a general feature, all PL decays are non-exponential, irrespective of the sample and excitation or/and emission wavelength which is expected given the multicentre structure of Eu3+ in ceria samples with strongly overlapped excitation and emission spectra.
Figure 8a gathers the PL decays of CE samples for excitation into the CT band and emission fixed at 591 nm. Such excitation and emission conditions favour the emission of the cubic Eu3+ centre. It is noteworthy to remark the absence of a notable emission quenching with Eu3+ concentration with the average PL lifetimes (Eq. 2) varying between 0.9 and 1.2 ms. Figure 8b compares the PL decays corresponding to emission of perturbed and cubic Eu3+ centres in CE(ow)-10 with excitation into the CT band. The PL decay of the cubic centre (emission wavelength fixed at 591 nm) is generally longer compared to perturbed centre (emission fixed at 580, 595 and 632 nm) which is related to the different radiative transition probabilities of the main emissions. Finally, Fig. 8c gathers the PL decays of the perturbed Eu3+ centres (emission wavelength set at 580, 632 and 713 nm) with excitation at 466 nm.
a Comparison between the PL decays of CE(cit) and CE(ow). Excitation was performed into the CT band and emission was measured around 591 nm (corresponding mainly to emission of the cubic Eu3+ centre); b Dependence of the PL decays of CE(ow)-10 on emission wavelength. Excitation was performed into the CT band and emission was measured around 580, 591, 595 and 632 nm; c Comparison between the PL decays of CE(ow)-10. Excitation was done at 466 nm, and emission was corresponding to the emission of perturbed Eu3+ centre
The results evidence also the predominant contribution of the main low symmetry Eu3+ centre despite that the co-existence of several minor low symmetry centres is clearly revealed from site selective excited emission spectra (Fig. 7).
Conclusions
Ceria doped with Eu3+ obtained by micro-emulsion or citrate methods or purchased were investigated using X-ray Diffraction, DR–UV/Vis, steady-state and time-resolved photoluminescence and Raman spectroscopies. Eu3+ was added via wetness impregnation on the preformed ceria nanoparticles in concentration of 1, 5 and 10 wt%, and the obtained samples were calcined in air at 1000 °C. Except for the commercial ceria, a remarkable homogeneity of Eu3+-ceria solid solutions was measured, even for the greatest Eu3+ concentration of 10 wt%. Overall emission properties agreed with a intrinsic structure composed of one cubic centre and four low symmetry centres (one main, three minor). It was also established from the emission decays that the concentration quenching was not significant for the isolated Eu3+ centres.
References
Gerhardt-Anderson R, Nowick AS (1981) Ionic conductivity of CeO2 with trivalent dopants of different ionic radii. Solid State Ionics 5:547–550
Kumar A, Babu S, Karakoti AS, Schulte A, Seal S (2009) Luminescence properties of europium-doped cerium oxide nanoparticles: role of vacancy and oxidation states. Langmuir 25:10998–11007
Sahu T, Singh Bisht S, Ranjan Das K, Kerkar S (2013) Nanoceria: synthesis and biomedical applications. Curr Nanosci 9:588–593
Yan CH, Sun LD, Liao CS, Zhang YX, Lu YQ, Huang HS, Lu SZ (2003) Eu3+ ion as fluorescent probe for detecting the surface effect in nanocrystals. Appl Phys Lett 82:3511–3513
Reisfeld R, Zigansky E, Gaft M (2004) Europium probe for estimation of site symmetry in glass films, glasses and crystals. Mol Phys 102:1319–1330
Fujihara S, Oikawa M (2004) Structure and luminescent properties of CeO2: rare earth (RE = Eu3+ and Sm3+) thin films. J Appl Phys 95:12
Choudhury B, Choudhury A (2012) Lattice distortion and corresponding changes in optical properties of CeO2 nanoparticles on Nd doping. Curr Appl Phys 13:217–223
Liu X, Chen S, Wang S (2007) Synthesis and photoluminescence of CeoO2:Eu3+ phosphor powders. J Lumin 127:650–654
Wang Z, Quan Z, Lin J (2007) Remarkable changes in the optical properties of CeO2 nanocrystals induced by lanthanide ions doping. Inorg Chem 46:5237–5242
Babu S, Schulte A, Seal S (2008) Defects and simmetry influence on visible emission of Eu doped nanoceria. Appl Phys Lett 92:123112
Li L, Yang K, Moon BK, Fu Z, Guo C, Jeong JH, Yi SS, Jang K, Lee HS (2009) Photoluminescence properties of CeO2:Eu3+ nanoparticles synthesized by a sol–gel method. J Phys Chem 113:610–617
Zeng L, Chen D, Cui L, Huang F, Wang Y (2010) High-content bulk doping and thermal stability of rare earth ions in CeO2 nanocrystals. Scipta Materialia 63:661–664
Tiseanu C, Parvulescu VI, Boutonnet M, Cojocaru B, Primus PA, Teodorescu CM, Solans C, Sanchez-Dominguez M (2011) Surface versus volume effects in luminescent ceria nanocrystals synthesized by an oil-in-water micro- emulsion method. Phys Chem Chem Phys 13:17135–17145
Tiseanu C, Parvulescu VI, Sanchez-Dominguez M, Boutonnet M (2012) Temperature induced conversion from surface to “bulk” sites in Eu3+- impregnated CeO2. J Appl Phys 112:013521
Chae KW, Park TR, Cheon CI, Cho NI, Kim JS (2013) Enhanced phasemiscibility and luminescence by inducing oxygen vacancies in Ce1−x Eu x O2- under a strongly reducing atmosphere. J Lumin 136:109–116
Sanchez-Dominguez M, Boutonnet M, Solans C (2009) A novel approach to metal and metal oxide nanoparticle synthesis: the oil-in-water micro- emulsion reaction method. J Nanopart Res 11:1823–1829
Courty P, Ajot H, Marcilly C, Delmon B (1973) Oxydes mixtes ou en solution solide sous forme très divisée obtenus par décomposition thermique de précurseurs amorphes. Powder Technol 7:21–38
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in Halides and Chaleogenides. Acta Cryst 32:751–767
Corma A, Atienzar P, Garcia H, Chane-Ching JY (2004) Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat Mater 3:394–397
Bensalem A, Muller JC, Bozon-Verduraz F (1992) Faraday communications. From bulk CeO2 to supported cerium–oxygen clusters: a diffuse reflectance approach, J. Chem Soc, Faraday Trans. 88:153–154. doi:10.1039/FT9928800153
Fornasiero P, Speghini A, Di Monte R, Bettinelli M, Kašpar J, Bigotto A, Sergo V, Graziani M (2004) Laser-excited luminescence of trivalent lanthanide impurities and local structure in CeO2–ZrO2 mixed oxides. Chem Mater 16:1938–1944
Tiseanu C, Cojocaru B, Parvulescu V, Sanchez-Dominguez M, Primus PA, Boutonnet M (2012) Order and disorder effects in nano-ZrO2 investigated by micro-Raman and spectrally and temporarily resolved photoluminescence. Phys Chem Chem Phys 14(37):12970–12981. doi:10.1039/c2cp41946g
Tiseanu C, Parvulescu V, Cojocaru B, Pemartin K, Sanchez-Dominguez M, Boutonnet M (2012) In situ Raman and time-resolved luminescence investigation of local structure of ZrO2 in the amorphous to crystalline phase transition. J Phys Chem C 116:16776–16783
Spanier JE, Robinson RD, Zhang F, Chan SW, Herman IP (2001) Size-dependent properties of CeO2−y nanoparticles as studied by Raman scattering. Phys Rev B 64:245407
McBride JR, Hass KC, Poindexter BD, Weber WH (1994) Raman and x-ray studies of Ce(1−x) RE(x) O(2−y), where RE = La, Pr, Nd, Eu, Gd, and Tb. J Appl Phys 76:2435–2441
Zhang F, Chan S-W, Spanier JE, Apak E, Jin Q, Robinson RD, Herman IP (2002) Cerium oxide nanoparticles: size-selective formation and structure analysis. Appl Phys Lett 80:127–129
Hernandez WY, Centeno MA, Romero-Sarria F, Odriozola JA (2009) Synthesis and characterization of Ce1−x EuxO2−x/2 mixed oxides and their catalytic activities for CO oxidation. J Phys Chem C 113:5629–5635
Dilawar N, Mehrotra S, Varandani D, Kumaraswamy BV, Haldar SK, Bandyopadhyay AK (2008) A Raman spectroscopic study of C-type rare earth sesquioxides. Mat Characterization 59:462–467
Eilers H, Tissue BM (1996) Laser spectroscopy of nanocrystalline Eu2O3 and Eu3+:Y2O3 Chem. Phys. Lett. 251:74–78
Görller-Walrand C, Fluyt L, Ceulemans A, Carnall WT (1991) Magnetic dipole transitions as standards for Judd–Ofelt parameterization in lanthanide spectra. J Chem Phys 95:3099–3106
Oomen EWJL, Van Dongen AMA (1989) Europium (III) in oxide glasses: dependence of the emission spectrum upon glass composition. J Non-Cryst Solids 111:205–213
Tiseanu C, Parvulescu V, Sanchez Dominguez M, Boutonnet M (2012) Temperature induced conversion from surface to bulk sites in Eu3+- impregnated CeO2. J Appl Phys 112:013521
Acknowledgements
Avram D, Cojocaru B and Tiseanu C acknowledge the Romanian National Authority for Scientific Research(CNCS-UEFISCDI) (project number PN-II-ID-PCE-2011-3-0534) for the financial support. Tiseanu C and Florea M also acknowledge COST Action CM1104 ‘Reducible oxide chemistry, structure and functions’.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Avram, D., Rotaru, C., Cojocaru, B. et al. Heavily impregnated ceria nanoparticles with europium oxide: spectroscopic evidences for homogenous solid solutions and intrinsic structure of Eu3+-oxygen environments. J Mater Sci 49, 2117–2126 (2014). https://doi.org/10.1007/s10853-013-7904-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7904-6