Abstract
Nano-hydroxyapatite (HA) was grown on functionalized multiwalled carbon nanotubes (MWCNTs) deposited on TiO2 nanofibers (NFs) that were hydrothermally grown on Ti metal sheets. The HA was electrochemically grown on the MWCNTs/TiO2 porous layer. It was found that the HA grows on the MWCNTs/TiO2 NFs in the form of dense coating with nanorice grain-shaped. The incorporation of MWCNTs between HA and TiO2 NFs has led to higher adhesion strength as measured by micro-scratching test indicating the benefit of MWCNTs on the improving the bonding strength of HA layer. The obtained coatings exhibit excellent corrosion resistance in simulated body fluid. It is expected that this simple route for preparing the new HA/MWCNTs/TiO2/Ti-layered structure might be used not only in the biomedical field, but also in catalysis and biological sensing among others.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium and titanium alloys are widely used to fabricate dental and orthopedic implants due to their high mechanical strength and toughness, excellent corrosion resistance in physiological environment, and good biocompatibility [1–3]. However, the bioinertness of the metallic surfaces inhibits the growth of bone tissue [4]. In order to overcome this shortage, hydroxyapatite (HA, Ca10(PO4)6(OH)2) has been applied as a coating on the metallic implants due to its similarity in chemical compositions, high biocompatibility, and osteoconductivity to bone tissue of human [5–8].
Carbon nanotubes (CNTs) are of particular interest in various applications as they exhibit high chemical and thermal stability, mechanical strength, flexibility, electrical and thermal conductivity as well as low density [9–11]. Recently, the unique biological and medical properties of CNTs have attracted considerable attention, and it is expected that biomaterials using CNTs are very promising as nanobiomaterials [12]. To optimize the use of CNTs, it is often necessary to assemble nanostructures onto their surface. The combination of CNTs and nanoparticles are expected to deliver exceptional performances in biomaterials [13, 14]. In general, the most of these nanocomposites used in biomaterials are TiO2 nanoparticles linked to or coated on CNTs [15, 16]. Nowadays, sol–gel coating followed by a heat treatment at elevated temperatures is the most commonly adopted method to introduce inorganic phases onto the CNT substrate [17, 18]. For instance, the heat treatment required in sol–gel processes often causes phase detachment between the TiO2 surface and underneath CNTs, agglomeration among neighboring TiO2/CNT cables and nonuniform crystallite. In all cases, the essential problem to be solved is the way to obtain a corrosion-resistant bioactive implant with better mechanical and biological properties and with good adhesion between HA coating and its substrate, especially those to be positioned in contact with bone like active prosthesis or functional matrices, etc. [4].
Recent reports indicated that modified Ti surface with high surface area of 1D TiO2 nanostructure films brought about by the small particle size is beneficial to show better mechanical interlocking between HA and Ti than conventional micro-roughened Ti surface. It was also shown that the addition of CNTs between Ti and HA layers increases the hardness, elastic modulus, and interlaminar shear strength of the HA/CNT/Ti layers [19]. In this context, dense layer of HA–CNTs coatings were successfully developed on Ti substrate coated with TiO2 nanotubes [20], using sol–gel method. Other studies have reported that nanostructured HA coatings have high chemical homogeneity, reduced flaw size, microstructural uniformity [21]. Moreover, the nanoscale roughness and grains as well as the high volume fraction of grain boundaries in nanostructured HA coatings can increase osteoblasts adhesion, proliferation, and mineralization [19, 21]. The combination of multiwalled carbon nanotubes (MWCNTs) and HA might be applicable not only in biomedical fields, but also in catalysis, biological sensing, and others [22, 23]. As a matter of fact, it would be interesting to combine mechanical and biological properties of the individual materials (Ti, TiO2, CNTs, and HA) layer by layer to obtain a single material with higher mechanical and biological properties.
This work reports on the preparation and characterization of a system consisting of HA layer with a novel nanorice grain-shaped, grown on MWCNTs/TiO2 nanofibers/bulk Ti-layered structure, using low temperature simple methods. To the best of our knowledge this is the first time that HA, in the form of nanorice, is deposited on a highly porous nanostructure consisting of TiO2 nanofibers (NFs) that were grown on Ti and coated with functionalized MWCNTs.
Experimental
Materials
Preparation of anatase TiO2 NF films
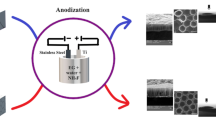
Pure titanium specimens with sizes 15 × 20 × 3 mm3 were mechanically polished, then treated by sonicating in ethanol and pickling in a 5 wt% oxalic acid solution at 100 °C for 2 h, followed by rinsing with distilled water and drying. A cleaned and homogeneous coarse surface of Ti samples was obtained. The obtained specimens were soaked in a mixed solution of 16 ml H2O2 (30 wt%) and 16 ml NaOH (10 M) in a Teflon autoclave. The autoclave was maintained at 80 °C for 24 h. After the autoclave was cooled to room temperature, the Ti plate was gently rinsed with distilled water. Thereafter, the as-prepared Ti sample was protonated through two cycles of ion exchange in 50 ml 0.1 M HCl for 2 h, and subsequently taken out, rinsed to neutral with distilled water, dried at 80 °C for 1 h, and finally calcined at 400 °C for 1 h to obtain anatase NF films on the Ti surface.
Functionalization and dispersion of MWCNTs
The functionalization and dispersion experiments are carried out on functionalized MWCNTs. The detailed preparation process was as follows: (i) MWCNTs were prepared then functionalized by oxidation in a mixture solution of HNO3+HCl in order to obtain ammonium salt of carboxylated carbon nanotubes (MWCNTs–COONH4). (ii) A 0.2 mg of functionalized MWCNTs was dispersed in a mixture consisting 0,02 mg polyvinylpyrrolidone and 40 ml distilled water, under ultrasonic treatment during 4 h.
Impregnation of TiO2 with MWCNTs
The Ti sheets coated with TiO2 NF film were introduced in a sealed container and kept under vacuum (10−2 mbar) for 15 min, then the solution containing the dispersed MWCNTs was injected on the Ti sheet surface by opening a valve which separates the sealed container from the solution. After that, the samples were gently rinsed with distilled water and dried at 80 °C. During this process many MWCNTs were entrapped in the TiO2 NF network.
HA deposition
Electrodeposition of HA was conducted at room temperature in an electrolyte containing 0.04 mol/l CaCl2, 0.027 mol/l (NH4)2HPO4, and 3 % H2O2. Thus, this solution is in Ca to P molar ratio of 1.67 with a pH value adjusted to 6.5, which is near to body condition. The deposition was carried out by applying a constant potential of 3 V. The solution was stirred during electrodeposition to obtain a uniform electrolyte concentration. The obtained coating was further treated in 0.1 mol/l NaOH solution at 80 °C for 2 h. Then the coated titanium cathodes were rinsed with distilled water and dried at 80 °C for 1 h. The prepared layered structure (HA/MWCNTs/TiO2 NFs/Ti) is sketched in Fig. 1.
Characterization methods
The surface morphology of TiO2 NFs, MWCNTs/TiO2 NFs, and HA/MWCNTs/TiO2 NFs were observed by means of scanning electron microscopy (FE-SEM, Nova nano SEM 630) and transmission electron microscope (TEM, Titan). X-ray photoelectron spectra (XPS) studies were carried out in a Kratos Axis Ultra DLD spectrometer equipped with a monochromatic Al Kα X-ray source (hν = 1486.6 eV) operating at 150 W, a multi-channel plate and delay line detector under vacuum of 1.0 × 10−12 bar. The survey and high-resolution spectra were collected at fixed analyzer pass energies of 160 and 20 eV, respectively. The instrument work function was calibrated to give an Au 4f 7/2 metallic gold binding energy (BE) of 83.95 eV and the spectrometer dispersion was adjusted to give a BE of 932.63 eV for metallic Cu 2p 3/2. To avoid differential charging the samples were mounted in floating mode. The crystallinity and the structure of the HA coating was examined using X-ray diffractometry (Bruker D-8 Advance powder diffractometer). Data were collected using Cu Kα X rays at 40 kV and 30 mA. The coatings were also characterized by Raman spectroscopy and Fourier transform infrared FT-IR. The Raman spectra were recorded by a LAB RAM ARAMIS using cobalt laser source, and the Fourier transform infrared traces were obtained by using a Nicolet 6700 FT-IR system with continuum IR microscope in the wave number range of 600–4000 cm−1. The scratch tests were performed by using a Zwick testing machine of materials equipped with microindentor. Cyclic polarization data were carried out using an EG&G 273 potentiostat (263A), the obtained data are in the form of potential versus logarithmic current density curves. A conventional three-electrode set up was used for electrochemical measurement, with graphite as counter electrode and standard Ag/AgCl as reference electrode). The potential was increased at a rate of 5 mV/s, within a scan range of +3 V to −2 V. The corrosion parameters (I corr, E corr, and R p) were calculated using 352SoftCorr III software.
Corrosion tests
Corrosion tests were carried out on uncoated and HA/MWCNTs coated TiO2 NFs films that are fixed in a cell exposing an area 1 cm2 in SBF solution at 37 °C. The SBF solution has the following ions concentrations: NaCl: 7.934 g/l; NaHCO3: 0.350 g/l; KCl: 0.222 g/l; K2HPO4: 0.174 g/l; MgCl2·6H2O: 0.303 g/l; CaCl2: 0.545 g/l; Na2SO4·10H2O: 0.161 g/l [24, 25].
Results and discussion
Scanning electron microscope images for TiO2 NFs before and after incorporation of the functionalized MWCNTs are shown in Fig. 2. Figure 2a, b shows SEM micrographs of TiO2 NFs without incorporation of MWCNTs. It reveals that the treated Ti surface is well covered with an obvious network of TiO2 NFs like bundles having an average diameter of about 50 nm. Actually, these bundles are constituted of NFs with very small diameter (about 10 nm) as revealed by the TEM image of Fig. 3. The electron diffraction pattern presented in Fig. 3b was taken from fibers shown in Fig. 3a. It exhibits ring diameters equal to those corresponding to the crystalline anatase TiO2 phase. Figure 2c, d shows SEM images of the MWCNTs/TiO2 nanostructure, which show well dispersed many MWCNTs with an average diameter of about 20 nm and a length of several micrometers. It is clearly seen that the MWCNTs are homogenously attached onto the surface of the TiO2 NFs. Also, it can be observed from these micrographs that the MWCNTs are bridged over the nanoporous structure, formed by the TiO2 NFs.
HRTEM of single TiO2 NF (Fig. 3c) shows well-crystallized anatase TiO2 NFs, but one can observe that the surface of the NFs is less crystallized than the inside due to the defects induced by chemical treatments used in its preparation. This irregular surface can increase the reaction between TiO2 NFs and MWCNTs.
The Raman spectra of TiO2 NFs and MWCNTs/TiO2 NFs are shown in Fig. 4. The Raman spectrum of TiO2 NFs shows clear signatures at about 397.5, 416.6, and 641 cm−1, as expected [26]. While the Raman spectrum of MWCNTs/TiO2 NFs shows both the features of TiO2 and the functionalized MWCNTs. For COONH4 functionalized MWCNTs, the Raman features observed at about 1363 and 1586 cm−1 represented the disorder-induced D band and graphite carbon-related G band, respectively [27, 28]. The peak that is observed around 1620 cm−1, as a shoulder to the G band, can be caused by the oxidation of MWCNTs. In addition, the intensity of the all Raman peaks is considerably enhanced in the case of MWCNTs/TiO2 NFs. This may be explained by the existence of a small Surface Enhanced Raman Scattering effect which may appear as a result of the MWCNT incorporation.
FT-IR spectra of MWCNTs/TiO2 NFs and pure TiO2 NFs are presented in Fig. 5. The two spectra showed a similar shape. For MWCNTs/TiO2 NFs, the band at 1550 cm−1 is attributed to the C=C bond which indicates the presence of MWCNTs. The band at 1714 cm−1 corresponds to C=O stretching of carboxylic groups [26], which confirms that the MWCNTs, integrated with the TiO2 NFs, have been fully functionalized. This is in accordance with the results of Raman spectra described above. Both TiO2 NFs and MWCNTs/TiO2 NFs have the same broad peak near the 3400 cm−1 corresponding to the stretching vibrations of O–H linkage on the TiO2 surface [29, 30]. As one can observe, the bands located at 1132 and 1296 cm−1 which correspond to TiO2 related vibrations, shift to lower binding energies when MWCNTs are incorporated. This may be due to the interaction between TiO2 and CNTs.
XPS were acquired to confirm the MWCNTs integration with TiO2 NFs. The XPS data from Ti, O, C, and N core levels of the MWCNTs/TiO2 NF surface are shown in Figs. 6 and 7. Figure 6 shows the core-level BE values of bulk TiO2 NFs; Ti 2p 3/2 at 458.3 eV and the Ti 2p 1/2 at 464 eV with spin-orbital splitting of 5.7 eV). But both Ti 2p 3/2 and Ti 2p 1/2 energies, measured from the MWCNTs/TiO2 surface are shifted to higher binding energies by 0.5 eV. This shift may be due to the formation of CO–Ti (OH)3 species that is explained by the chemical reactions below. The hydroxide group (OH) is more electronegative than CO group, so it interacts with the Ti atom by, either capturing its electrons or causing its polarization, thus leading to the rise of the BE. Since most metals are less electronegative than OH group, the formation of a hydroxide should be registered in XPS by an increase in BE of the Ti peak. Moreover, the O/Ti atomic ratio is calculated to be about 2.4 (Table 1), which is higher than the corresponding ratio for pure TiO2 (Table 2). This may stem from the oxygen related to the oxidized MWCNTs that was attached to the Ti ions of TiO2. Figure 7 shows the corresponding high-resolution XPS and curve fittings of C 1s, O 1s and N 1s spectra where Fig. 7a, b present the high-resolution XPS data from C 1s core levels of the TiO2 NFs before and after impregnation with MWCNTs, respectively. The C 1s peaks from the TiO2 NFs before impregnation (Fig. 7a) can be fitted to four peaks. The BE of the contaminant carbon on the surface is assigned at 284.8 eV (C–C/C=C bonds). The three peaks with binding energies located at 284, 286.2, and 288.7 eV can be assigned to C–H, C–O, and C=O species, respectively [31–33]. After MWCNT integration, the C 1s band, considerably, increased in intensity and can be fitted to six peaks (Fig. 7b). The BE of MWCNTs and the contaminant carbon is assigned at 284.7 eV (C–C/C=C bonds). The four peaks with binding energies located at 283.5, 286.4, 288, and 289.8 eV can be assigned to C–H, C–O, C=O, and O–C=O species, respectively. The presence of the O–C=O species confirms that the MWCNTs, integrated into the composite, have been functionalized with carboxyl groups. The sixth peak appearing at about 285.6 eV may be attributed to the presence of CO/Ti(OH)3 structure resulting from the interaction between the functionalized MWCNTs and TiO2 NFs [34]. This peak confirms the linkage between the TiO2 NFs and the MWCNTs.
Similarly, the O 1s spectrum of the TiO2 NFs (Fig. 7c) can be fitted to four peaks located at 529.6, 531, 532, and 532.5 eV and attributed to TiO2 NFs, OH [35], C=O, and C–O, respectively. After MWCNT integration, the O 1s peaks can be fitted to five peaks with four peaks located at 530.8, 532.3, 532.7, and 533.5 eV and attributed to TiO2 NFs, C–O, C=O, and O–C=O, respectively [31]. The fifth peak located at 531.6 eV is assigned to the CO/Ti(OH)3 structure [31]. These results provide further evidence that oxygen-based functional groups were linked to the TiO2 NFs network. As shown by the deconvoluted O 1s core level spectrum before and after MWCNTs impregnation (Fig. 7c, d), the fitted peak related to the TiO2 NFs is shifted with 1.3 eV in the same direction of Ti 2p shift (mentioned above). The shift may be due to contribution from two effects: (1) the chemical shift related to OH attachment to Ti as explained above, (2) EF (Fermi level) shift which causes apparent rigid BE shift in the same direction irrespective of the atomic species involved, since the BE, by definition, is given as the energy difference from the E F [36, 37]. The high-resolution peak of N 1s (400.5 eV) can be attributed to the presence of pyrrolic/pyridine nitrogen [33] (Fig. 7d) resulting from MWCNT functionalization.
The possible interaction between the functionalized MWCNTs and TiO2 NFs could be interpreted by means of chemical interaction between the modified MWCNTs and TiO2 NFs. In our experiment we used ammonium salt of carboxylated carbon nanotubes (MWCNT–COONH4) to create chemical compatibility with the TiO2 NFs surface. Such surface functional groups (O=C–O or COO−) provide active sites for directed reaction between anatase nanocrystals and MWCNTs [36]. For example, the –OH groups on the Titanium possibly react with the –OH and –COOH groups on the MWCNTs surface through esterification to form C–O–Ti or O=C–O–Ti bonds [38]. In this study, the chemical reactions between TiO2 and MWCNTs–COONH4 can be expressed as follows.
Initially, transition metal are, usually, highly reactive toward hydrolysis reaction which must take place first and form Ti hydroxide; Ti (OH) 4 [32]. The layer in contact with air submits hydrolysis reaction (1).
In water the resultant molecule decomposed to [Ti(OH)3] + with delocalized charge and a very small amount of Ti+ (OH)3 with localized charge on Ti atoms.
The used electrolyte R–COONH4 (MWCNTs–COONH4) dissociates strongly in water even more than R–COOH (MWCNTs–COOH) by reaction 3:

 reacts with the Ti+ (OH)3 in the solution as shown in Scheme 1.
reacts with the Ti+ (OH)3 in the solution as shown in Scheme 1.
There is an equilibrium reaction between [Ti(OH)3]+ and Ti+ (OH)3 as shown in reaction (4).
K is the constant of instability \( (K = \frac{x}{{n_{0} - x}}) \) and in order to keep the equilibrium, the molecules \( {\text{Ti}}^{ + } ({\text{OH}})_{3} \) react with  leading to a transformation of [Ti(OH)3]+ to Ti+ (OH)3.
leading to a transformation of [Ti(OH)3]+ to Ti+ (OH)3.
A HA layer was deposited on the TiO2 NFs/Ti and MWCNTs/TiO2/Ti substrates through an electrodeposition process. The SEM images of the HA coating shown in Fig. 7 reveal a continuous and uniform HA coating with nanorice particle morphology for the two substrates; TiO2 NFs (Fig. 8a, b) and HA/MWCNTs/TiO2 structure (Fig. 8c, d). Compared to the HA layer deposited on the TiO2 NFs only, the HA layer deposited on the TiO2 impregnated with functionalized CNTs shows dense coating with nanorice particles, which may lead to higher adhesion strength and good corrosion resistance. To study the crystal structure of the nano-HA deposited on MWCNTs/TiO2/Ti substrate, we analyzed the XRD pattern of electrodeposited coatings before and after alkaline treatment. The XRD pattern (Fig. 9a) of the electrodeposited coating mostly consisted of calcium hydrogen phosphate (CaHPO4·2H2O, CHP) which transformed to HA in alkaline solution. The intense diffraction peaks shown in Fig. 9b at 25.84° and 32° belong to (002) and (211) crystal planes of HA coating, and the peaks at 33.26°, 34.17°, 35.19° also came from HA. The diffraction peak located at 25. 3° is attributed to the anatase TiO2 NFs.
In order to improve the crystallization and the interdiffusion between TiO2 NFs and HA nanorice and relax the electrodeposition stresses [39], the samples were annealed in air at 900 °C for 1 h. The XRD pattern of the annealed samples (Fig. 9c), obviously, shows better crystallization of HA coating with sharp diffraction peaks, and anatase transformation to rutile with disappearance of the Ti peaks. This indicates that not only all the anatase transformed into rutile, but part of the Ti substrate, also, has oxidized to the rutile phase.
FT-IR spectrometry of the HA coating, after annealing, is shown in Fig. 10. Phosphate (PO4 3−) ν3 vibration is found in the band region of 1065–1160 cm−1 while phosphate ν1 band is present at 973 cm−1 [40]. The peaks in the region of 1377 and 1458 cm−1 are due to ν3 vibration mode of carbonate CO3 2− ion, indicating that part of calcium in HA transformed to CaCO3 upon annealing. OH− composing HA related band is observed at about 1631 cm−1. There are faint absorptions at about 870 and 1226 cm−1, which belong to HPO4 2− bands [40]. Significant bands are also discerned between 2700 and 3050 cm−1 that are due to adsorbed water. The characteristics of the spectrum are similar to those of mineral phase of bones [41].
Raman spectrum (Fig. 11a) from as-deposited HA/TiO2/Ti film exhibits bands at 142, 435, 593, 966, and 1075 cm−1. The first band corresponds to anatase NFs film that may originate from the under layer TiO2. The strongest band at 966 cm−1 corresponds to the symmetric stretching mode ν1 P–O of the tetrahedron PO4 3− [42]. The other bands observed in the frequency regions of 400–490, 570–625, and 1020–1075 cm−1 are assigned to the ν2, ν4, and ν3 type of internal PO4 3− mode [42], respectively. Raman spectra of HA/TiO2/Ti and HA/MWCNTs/TiO2/Ti annealed at 900 °C (Fig. 11b, c) show all these bands along with TiO2 rutile bands (442 and 606 cm−1), in addition to the frequency band situated at 230 cm−1 which corresponds to the TiO2 rutile structure. From these spectra, one can observe that the rutile bands in the HA/TiO2/Ti heat treated at 900 °C are strongest than the rutile bands observed in the HA/MWCNTs/TiO2/Ti heat treated at the same temperature (Fig. 11c). On the other hand, the strongest peak which corresponds to the HA structure is very weak in the case of the HA/TiO2/Ti film in comparison with the observed one in the HA/MWCNTs/TiO2/Ti layer. This indicates, also, that the HA coating on the MWCNTs/TiO2 film is more dense and abundant than the HA coating on the TiO2 NFs alone.
In order to confirm the effect of the multiwalled CNTs on the adhesion strength of the HA layer, we used scratching method that consists in moving an indenter (with pyramidal shape) on the surface of the specimens under an initial constant force (20 N). The applied force produces a damage of the film whose extent depends on the adherence. Clearly, it appears that MWCNT impregnation can improve the HA adhesion as can be seen in Fig. 12a, b. On the other hand, heat treatment at 900 °C under air can further enhance the adhesion strength as shown in Fig. 12c, d.
Metallic implants may be susceptible to corrosive attack by body fluids with subsequent release of metallic ions which might cause adverse effects to the surrounding tissues. Therefore, it is crucial to investigate the corrosion behavior of implant materials interacting with physiological environment. Figure 13 shows cyclic potentiodynamic polarization curves for TiO2 NFs/Ti and HA/MWCNTs/TiO2 coated Ti substrate. The corrosion parameters extracted from these curves (using special software: 352 soft Corr III) are shown in Table 3. It can be seen that both samples have very low anodic current density (I corr), compared to pure Ti foil (34.66 μA/cm2) and nano-HA films (100 nA/cm2) prepared by using electrophoretic method [42]. Icorr values of the TiO2 NFs/Ti and HA/MWCNTs/TiO2 coatings are 0.044 and 0.025 nA/cm2, respectively. Compared to other works [40, 42], these anodic corrosion currents are almost negligible and present the lowest corrosion rate. One can observe, also, that HA-coated sample has the lowest current density and the highest corrosion resistance which is two times higher than that of TiO2 NFs on Ti substrate. In addition, the anodic and cathodic potentiodynamic curves of the HA-coated sample are superposed with a potential of almost 0 mV (Fig. 13b) which is a further evidence of more stability. However, the anodic and cathodic curves corresponding to the TiO2 NFs alone are not superposed, but the potential shifted towards noble metallic potential direction and reaches 260 mV (Fig. 13a). Moreover, no one of the tested sample exhibits appreciable hysteresis indicating the absence of passivity breakdown. Consequently, the coatings, effectively, act as a well barrier to the transport of electrons and ions between the substrate and the SBF solution.
Conclusions
HA/MWCNTs/TiO2 coatings were successfully fabricated on pure Ti substrate. Before coating, the Ti surface was chemically patterned with highly porous TiO2 NFs network. Functionalized MWCNTs were successfully integrated between HA and TiO2 NFs and showed a certain role in anchoring the HA coating. The microstructure analysis of HA/MWCNTs/TiO2 surface showed homogeneous and dense nanorice particle morphology in comparison with the HA layer deposited on TiO2 NFs only, which led to higher adhesion strength of the coating. The adherence can be further improved by annealing and the obtained coatings show very good resistance corrosion in SBF solution.
References
Long M, Rack HJ (1998) J Biomater 19:1621
Wang ZW, Wang YM, Liu Y, Xu JL, Guo LX, Zhou Y, Ouyang JH, Dai JM (2011) J App Phys 11:1405
Macwan DP, Dave PN, Chaturvedi S (2011) J Mater Sci 46:3669. doi:10.1007/s10853-011-5378-y
Afshar A, Yousefpour M, Yang XD, Li XD, Yang BC, Wu Y, Chen JY, Yang XD (2006) J Mater Sci Eng B 128:243
Shaylin S, George JD (2012) J Acta Biomater 8:20
Anselme K (2011) J Osteoporos Int 22:2037
Xiao XF, Liu RF, Zheng YZ (2006) J Surf Coat Tech 200:4406
Park JH, Lee DY, Oh KT, Lee YK, Kim KM, Kim N (2006) J Mater Lett 60:2573
Guohua J, Xiaoyan Z, Yin W, Tianwen L, Xinke S (2011) J Powder Technol 207:465
Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL (2006) J Crit Rev Toxicol 36:189
Jiang G, Wang L, Chen C, Dong X, Chen T, Yu H (2005) J Mater Lett 59:2085
Teresa Cristina OM, Tiago GS, Cristina PS, Evaldo JC, Fernanda RM, Anderson OL (2012) Langmuir 28:4413
Hsu CH, Liao HY, Kuo PL (2010) J Phys Chem C 14:7933
Kauffman DR, Tang Y, Kichambare PD, Jackovitz JF, Star A (2010) J Energ Fuel 24:1877
Suwilai C, Siwaporn M, Smith JS (2011) J Microsc Soc Thail 1:36
Bai Y, Park I, Bae T, Kim K, Watari F, Uo M, Lee M (2011) J Wuhan Univ Technol Mater Sci Ed 5:867
Baowan D, Triampo W, Triampo D (2009) New J Phys 11:093011
Liu B, Zeng HC (2008) J Chem Mater 20:2711
Kaya C (2008) J Ceram Int 34:1843
Ji Xiaoli, Weiwei L, Qi W, Jianfeng M, Haihong X, Qing B, Chuantong L, Jinsong L (2012) Int J Mol Sci 13:5242
Bokai Z, Chi TK (2011) J Mater Sci Mater Med 22:2249
Zhengjie L, Lei C, Zengchao Z, Yueyun L, Yunhui D, Yubing S (2013) J Mol Liq 179:46
Vassiliki K, Theodoros T, Theodora K, Antonios K, Konstantinos B, Jeff Th, Dimitrios G (2013) J Mater Sci Eng B 178:457
Zhu L, Ye X, Tang G, Zhao N, Gong Y, Zhao Y, Zhao J, Zhang X (2006) J Biomed Mater Res 78A(2006):515
Advincula MC, Petersen D, Rahemtulla F, Advincula R, Lemons JE (2007) J Biomed Mater Res Part B 80:107
Peining Z, Nair AS, Shengyuan Y, Ramakrishna S (2011) J Mat Res Bull 46:588
Frank O, Mohr M, Maultzsch J, Thomas C (2011) J ACS NANO 5:2231
Zhang J, Zou HL, Qing Q, Yang YL (2003) J Phys Chem B 107:3712
Wang H, Wang HL, Jiang WF (2009) J Chemosphere 75:1105
Chena L, Pang X, Yua G, Zhanga J (2010) J Adv Mater Lett 1:75
Kaciulis S, Mattogno G, Pandolfi L, Cavalli M, Gnappi G, Montenero A (1999) J App Surf Sci 151:1
Yan XB, Tay BK, Yang Y (2006) J Phys Chem B 110:25844
Achour A, Vizireanu S, Dinescu G, Le Brizoual L, Djouadi MA, Boujtita M (2013) J App Surf Sci 273:49
Wagner CD, Naumkin AV, Kraut-Vass A, Allison JW, Powell CJ, Rumble JR (2003) NIST Standard Reference Database 20, Version 3.5
Wang LQ, Baer DR, Engelhard MH, Shultz AN (1995) J Surf Sci 237:344
Min CH, Cho S, Lee SH, Cho DY, Park WG, Chung JG, Lee E, Lee JC, Anass B, Lee JH, Hwang CS, Oh SJ (2010) J Appl Phys Lett 96:201907
Achour A, Ait Aissa K, Mbarek M, El Hadj K, Ouldhamadouche N, Barreau N, Le Brizoual L, Djouadi MA (2013) J Thin Solid Films 538:71
Jiaguo Y, Tingting M, Shengwei L (2011) J Phys Chem Chem Phys 13:3491
Kar A, Raja KS, Misra M (2006) J Surf Coat Technol 201:3723
Mohan L, Durgalakshmi D, Geetha M, Sankara Narayanan TSN, Asokamani R (2012) J Ceram Int 38:3435
Ban S, Maruno S (1993) J Appl Phys 32(10B):1577
Rath PurnaC, Besra Laxmidhar, Singh BimalP, Bhattacharjee Sarama (2012) J Ceram Int 38:3209
Acknowledgements
The authors acknowledge Professor C. Sahraoui from King Abdullah University of Science and Technology (KAUST) for his assistance in microanalysis and funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chetibi, L., Achour, A., Peszke, J. et al. Hydroxyapatite growth on multiwall carbon nanotubes grown on titanium fibers from a titanium sheet. J Mater Sci 49, 621–632 (2014). https://doi.org/10.1007/s10853-013-7742-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7742-6