Abstract
The electronic and optical properties of Cu2ZnSnX4 (X = S, Se) have been studied using the highly accurate Tran–Blaha-modified Beck and Johnson (TB-mBJ) potential. From this study, it has been found that these compounds are direct band semiconductors and the value of band gap increases from Se to S. The values of the band gap, density of states, bulk modulus, dielectric functions, real part of optical conductivity, and absorption coefficient are reported. The calculated band gap value is found to be in excellent agreement with the recent experimental results. A comparison of the calculated properties with available experimental data indicates that the TB-mBJ approach provides a better description of the electronic and optical properties of the compound than the standard GGA approaches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper–zinc–tin–chalcogenides-based quaternary semiconductors have received wide attention as potential solar cell absorber materials. An ideal solar energy absorber should have a band gap around 1.5 eV and a high optical absorption coefficient for the visible light. Based on this fact, the band gaps and optical properties of the copper-based systems were extensively studied both experimentally [1–3] and theoretically [4, 5]. The band gap energy (E g) of Cu2ZnSnS4 (CZTS) and Cu2ZnSnSe4 (CZTSe) are around 1.0–1.5 eV, which is suitable for photovoltaic applications. Moreover, knowledge of the optical properties, such as the dielectric function and the optical absorption coefficient, is required to analyse optical measurements as well as to optimize the solar cell devices. Various research groups reported the electronic structure calculations [4–8]. But new approach, such as TB-mBJ, is introduced recently. Overall, TB-mBJ is a decent alternative for GW or hybrid functional, achieving a comparable accuracy at a far lower cost. The combination of TB-mBJ exchange and generalise gradient approximation (GGA) correlation produced accurate semiconductor gaps for numerous semiconductors and insulators [9–13]. In this paper, the full potential linearized augmented plane wave (FP-LAPW) method with the TB-mBJ potential has been used to investigate the electronic and optical properties of the copper-based solar cell materials.
Method of calculation
As a computational tool, for Cu2ZnSnX4 (X = S, Se), we have employed the full potential augmented plane wave method (APW + lo) based on density functional theory (DFT) using Vienna package WIEN2K code (FP-LAPW) [14]. We have used the GGA within the Perdew–Burk–Ezrenhof (PBE) parameterization [15] and TB-mBJ [9–11] potential. A plane wave expansion has been done with K max × R max equal to 7 and l max = 10 are kept constant throughout the calculations. The charge density is Fourier expanded up to G max = 12(Ry)1/2. A 2 × 2 × 2 division for k-point sampling is used, and the tetrahedral method [16] has been employed for the Brillouin zone integrations. The Zn (3d 10 4s 2), Cu (3p 6 4s 2 3d 9), Sn (4d 10 5s 2 5p 2), S (3s 2 3p 4), and Se (3d 10 4s 2 4p 4) orbital are treated as valence states. The muffin tin radii for Zn, Cu, Sn, S, and Se are chosen to be 2.25, 2.22, 2.48, 1.97, and 1.97 a.u., respectively, for stannite structure and for kesterite structure, the radii are 2.22, 2.34, 2.41, 1.91, and 1.91 a.u., respectively. A number of iterations (40) are dedicated to accomplish self consistency. Less than 0.00001Ry of the total energy difference is used as per formula unit for succeeding iterations.
The frequency-dependent complex dielectric function ε(ω) = ε 1(ω) + iε 2(ω) is known to describe the optical response of the medium at all phonon energies E = ℏω. The imaginary part of the ε 2(ω) in the long wavelength limit has been obtained directly from the electronic structure calculation, using the relation [17],
where M is the dipole matrix, i and j are the initial and final states, respectively, f i is the Fermi distribution function for the ith state, and E i is the energy of electron in the ith state. The real part of the dielectric function [ε 1(ω)] can be extracted using the Kramers–Kronig relation [18]
where p stands for the principal value of the integral. The knowledge of both the real and imaginary parts of the dielectric tensor allows the calculation of the important optical properties, such as absorption coefficient, electron energy loss function, and real part of optical conductivity.
The absorption coefficient, α(ω) and real part of the optical conductivity, Re[σ(ω)] are calculated using the relation as follows [19–21]:
Results and discussion
Structural properties
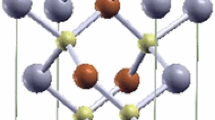
The crystalline and optical properties of CZTS and CZTSe have been discussed and compared with available literature results [1, 5]. The I2–II–IV–VI4 quaternary semiconductors have two fundamental crystal structures: one is the kesterite structure (KS) [space group \( I\bar{4} \)], the other is the stannite structure (SS) [space group \( I\bar{4}2m \)], which are shown in Fig. 1. In all of the structures, Se or S (group VI) atom is surrounded by two Cu (group I) atoms, one Sn (group IV) atom and one Zn (group II) atom, therefore, the octet rule is obeyed.
The calculated total energies within GGA as function of the volume were used for determination of theoretical lattice constant and bulk modulus. Equilibrium lattice constant, bulk modulus and its pressure derivative are calculated by fitting the calculated total energy to the Murnaghan’s equation of state given in equation
where E 0 is the minimum energy at T = 0 K, B 0 is the bulk modulus at the equilibrium volume, and B 0′ is pressure derivative of the bulk modulus at the equilibrium volume. The equilibrium volume is given by the corresponding total energy minimum as shown in Fig. 2a–d. The volume corresponding to the lowest energy is used in the determination of equilibrium lattice constant. The calculated values of lattice constant and bulk modulus are presented in Table 1. It is clearly seen that the structural stability of these compounds is more stable in kesterite than stannite structure. We have also optimized the positional parameter and the c/a ratio for all the compounds; it is found that the c/a ratio is very close to 2. The bulk modulus is slightly higher in KS when compared to SS for CZTS; whereas the bulk modulus is greater in SS when compared to KS for CZTSe. The calculated lattice constants for both KS and SS are in good agreement with the experimental values.
Density of states (DOS)
Figure 3a–d shows calculated partial and total density of states for all four CZTS and CZTSe. Overall, they have rather similar energy distribution of the eigenstates. The VB–DOS contains mainly a hybridization of Cu-d and anion (S and Se)-p, although there are also contributions from Zn-spd, Cu-s, and Sn-p in the lower energy region of the DOS (i.e., −6.5 to −2 eV). The CB-DOS consists mainly of cation-s anion-p hybridization. In the range between −2.5 and −4 eV below the Fermi level, Sn-p shows highest contribution. Within the range −1.28 to 1.5 eV around E F, Zn-s shows major contribution. On the other hand, in conduction band side anion, (S and Se)-p shows a peak in between 1.2 and 1.7 eV. So, the band gap is formed between the hybridized “p” like states of anion in the valence band and the antibonding states in the conduction band of Zn-s like states [1, 21].
By analyzing the partial density of these compounds, it is found that the s–p interaction modifies the gap of these materials. The anion-p like states are pushed up and Zn-s like states are pushed down. The bands above 2.5 eV mainly consists of the hybridised bands of Zn “s” like, Sn “p” like and anion “p” like bands. The energy gap is about 1.5 eV. The higher CBs do not contribute to the optical absorption in the low-energy regime. The calculated band gap values are reported in Table 1. Moreover, our calculated band gap values are in good agreement with earlier theoretical predictions and experimental investigations [1, 22–26].
Optical properties
The optical properties of the CZTX (X = S, Se) can be described by means of complex dielectric function ε(ω). The dielectric function describes the linear response of the system to an electromagnetic radiation, which is related to the interaction of photons with the electrons. The magnitude of optical properties depends on the polarization of photons. For the tetragonal unit cell structure, we calculate two independent components of dielectric constant ε(ω). One perpendicular component and the other parallel component, which arises due to polarization along the z direction. All the other optical constants, such as refractive index n(ω), the extinction coefficient k(ω), the optical reflectivity r(ω), and absorption coefficient α(ω), are then computed from the components of the dielectric function.
Figures 4 and 5a–d show the real and imaginary parts of the dielectric function. The real part of the dielectric function, ε 1(ω), represents the dispersion of the incident photons by the materials, while imaginary part of the dielectric function, ε 2(ω) is related to the energy absorbed by the material. The values of the static dielectric constant for a field perpendicular or parallel to the tetragonal c-axis are listed in Table 2. The dielectric constant ε 1(0) is obtained from the zero-frequency limit of ε 1(ω) and it corresponds to the electronic part of the static dielectric constant of the material. It is a parameter of fundamental importance in many aspects of materials properties.
From the application point of view, it is very interesting to determine the transition corresponding to each peak in the spectrum. The peak in the ε 2(ω) spectrum distributes in a sequence similar to that in density of states. The main difference of dielectric functions between TB-mBJ and GGA becomes noticeable in the energy range from 0 to 5 eV. Transitions from valence bands at top to conduction bands at the lower state contribute to the optical spectra’s major part [21]. The dramatically increasing point of the mBJ imaginary part ε 2 is shifted to higher photon energy with respect to that of the GGA ε 2. The rightward shift of the TB-mBJ ε 2 results between 2 and 5 eV with respect to the GGA ones reflects the fact that the TB-mBJ energy gap is substantially improved over the GGA one. The zero-frequency real part ε 1 of static dielectric functions and scissor operator are reported in Table 2, in comparison with earlier calculated value [1, 5, 27].
Furthermore, the dielectric functions of all four components have a strong ε 2(ω) peak in the energy range between 3.6 and 4.3 eV. This peak arises from the response at \( \overrightarrow {k} \)-states always from the Γ-point, with a strong contribution from the flat dispersion of the top most VB and of the lowest CB. The two kesterite compounds have more pronounced peaks around 1.6–2.0 eV, whereas the two stannite compounds show much stronger anisotropy of their response peaks. In the kesterite compounds, the upper VBs are energetically close together at 0–0.5 eV at the BZ edges, whereas the upper VBs in the stannite compounds have more split with energy in the region of about 0.5–1.0 eV. It is the symmetry of these VBs in combination with their energy dispersions that cause the anisotropy [1, 5].
The calculated real part of the optical conductivity (frequency dependent) using GGA and TB-mBJ potentials is shown in Fig. 6a–d. The material’s conductivity increases with an increase of energy from 3.3 to 9.03 eV. Here, the conductivity for both the structures of CZTS and CZTSe has the highest value. Afterwards, it decreases with the energy; higher energy implies that the material does not interact with incident photon.
The absorption band edges of CZTS_KS, CZTS_SS, CZTSe_KS, and CZTSe_SS with scissor correction using GGA and TB-mBJ are shown in Fig. 7. The band gap energies (E g) of CZTS and CZTSe were calculated from the Tauc’s plot assuming α 2 ∝ (hν − E g ), where α is the absorption coefficient and hν is the photon energy (h is the Planck’s constant and υ is the frequency). It is found that the theoretical absorption spectra of CZTS_KS and CZTSe_KS agree well with the available experimental spectra [1, 22, 28, 29] in their shape. The estimated band gaps using TB-mBJ including scissor correction are 1.58, 1.63, 1.34, and 1.55 eV for CZTS_KS, CZTS_SS, CZTSe_KS, and CZTSe_SS, respectively, (Fig. 7). All these values are quite close to the experimental value for a single-junction solar cell [28, 30–33]. The absorption coefficient was higher than 104 cm−1 in the visible region. Actually, the absorption coefficient, α(ω) of CZTS_KS, CZTS_SS, CZTSe_KS, and CZTSe_SS represent the linear optical response from the valence bands to the lowest conducting bands. All three components have comparable absorption, although with different photon energies for the onset to absorption (i.e., the band gap energy). The absorption starts in the energy of fundamental gap. In the energy range of 1.1–1.8 eV, the absorption peaks are due to the transition of electron in the hybridized states of Cu-d and anion-p to empty states in the CB. The value of α(ω) increases as the energy increases to its higher value. Therefore, it is worthy to investigate extensively CZTS and CZTSe for the sake of obtaining high-efficient materials.
Conclusion
In summary, we have used TB-mBJ exchange potential implemented in WIEN2K (FP-LAPW) method to investigate the electronic and optical properties of Cu2ZnSnX4 (X = S, Se). The calculated results show that the energy gaps are substantially improved by TB-mBJ over GGA values toward experimental results and also we found that the compounds are direct band gap semiconductors. The behavior of optical dielectric functions (both real and imaginary parts) and optical conductivity and absorption coefficients as functions of photon energy agree well with the experimental and other literature values. The present study reveals that these excellent improvements are achieved because TB-mBJ potential describes accurately the hybridization between the Zn-s and anion (S and Se)-p states.
References
Persson C (2010) J Appl Phys 107:053710
Suryawanshi MP, Agawane GL, Bhosale SM, Shin SW, Patil PS, Kim JH, Moholkar AV (2013) Mater Technol Adv Perform Mater 28:98
Walsh A, Chen Su, Wei S-H, Gong X-G (2012) Adv Energy Mater 2:400
Zhao Z, Chenshuo M, Cao Y, Juan Y, Xijia He, Qiu J (2013) Phys Lett A 77:417
Zhao H, Persson C (2011) Thin Solid Films 519:7508
Sevik C, Cagin T (2010) Phys Rev B 82:045202
Liu HR, Chen S, Zhai YT, Xiang HJ, Gong XG (2012) J Appl Phys 112:093717
Maeda T, Nakamura S, Wada T (2011) Jpn J Appl Phys 50:04DP07
Tran F, Blaha P (2009) Phys Rev Lett 102:226401
David K, Tran F, Blaha P (2012) Phys Rev B 85:155109
Shiyou C, Gong XG, Aron W, Wei S-H (2009) Appl Phys Lett 94:041903
Singh DJ (2010) Phys Rev B 82:155145
Singh DJ, Seo SSA, Lee HN (2010) Phys Rev B 82:180103
Blaha P, Schwarz K, Madsen GKH, Kvasnicka D, Luitz J (2001) WIEN2K an augmented plane wave + local orbitals program for calculating crystal properties, Karlheinz Schwarz, Techn. University at Wien, Austria ISBN 3-501031-1-2
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:386
Monkhorst HJ, Pack JD (1976) Phys Rev B 13:5188
Okoye CMI (2003) J Phys Condens Matter 15:5945
Amin B, Ahmad MI, Maqbool S, Said G, Ahmad R (2011) J Appl Phys 109:023109
Sun J, Wang H, He J, Tian Y (2005) Phys Rev B 71:125132
Ozaki S, Adachi S (1994) J Appl Phys 75:7470
Saha S, Sinha TP (2000) Phys Rev B 62:8828
Yakuphanoglu F (2011) Sol Energy 85:2518
Matsushita H, Maeda T, Katsui A, Takizawa T (2000) J Cryst Growth 208:416
Kamoun N, Bouzouita H, Rezig B (2007) Thin Solid Films 515:15
Katagiri H, Sasaguchi N, Hando S, Hoshino S, Ohashi J, Yokota T (1997) Sol Energy Mater Sol Cells 49:1
Zoppi G, Forbes I, Miles RW, Dale PJ, Scragg JJ, Peter LM (2009) Prog Photovolt 17:5
Joachim P, Ryoji A, Akihiro N, Georg K (2009) Phys Rev B 79:115126
Tanaka K, Oonuki M, Moritake N, Uchiki H (2009) Sol Energy Mater Sol Cells 93:583
Rajesh G, Muthukumarasamy N, Subramaniam EP, Agilan S, Velauthapillai D (2013) J Sol Gel Sci Technol. doi:10.1007/s10971-013-3006-9
Jiang M, Li Y, Dhakal R, Thapaliya P, Mastro M, Caldwell JD, Kub F, Yan X (2011) J Photonics Energy 1:019501
Katagiri H, Ihigaki N, Ishida T, Saito K (2001) Jpn J Appl Phys 40:500
Seol J, Lee S, Lee J, Nam H, Kim K (2003) Sol Energy Mater Sol Cells 75:155
Zhao Z, Chenshuo M, Yuechan C, Juan Y, Xijia H, Jianbei Q (2013) Phys Lett A 377:417
Todorov TK, Reuter KB, Mitzi DB (2010) Adv Mater 22:20
Oliveira TA, Coutinho J, Torres VJB (2013) Thin solid films 535:311
Babu GS, Kumar YBK, Bhaskar PU, Raja VS (2008) Semicond Sci Technol 23:8
Acknowledgements
The authors thank the Indian School of Mines, Dhanbad, India, for providing the Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, A., Thangavel, R. & Rajagopalan, M. First principles study of electronic and optical properties of Cu2ZnSnX4 (X = S, Se) solar absorbers by Tran–Blaha-modified Becke–Johnson potential approach. J Mater Sci 48, 8259–8267 (2013). https://doi.org/10.1007/s10853-013-7638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7638-5