Abstract
Ba0.85Ca0.15Ti0.90Zr0.10O3 + xmol% MnO2 lead-free ceramics have been prepared by a conventional sintering method and the effects of MnO2 and sintering temperature on microstructure, ferroelectric, and piezoelectric properties of Ba0.85Ca0.15Ti0.90Zr0.10O3 lead-free ceramics have been studied. The addition of 0.25 mol% MnO2 promotes grain growth, improves the ferroelectricity of the ceramics and strengthens ferroelectric tetragonal–ferroelectric orthorhombic phase transition near 40 °C. Because of the coexistence of tetragonal and orthorhombic phases and the combinatory effects of soft and hard doping of Mn ions, the ceramic with x = 0.25 exhibits the optimum piezoelectric properties (d 33 = 306 pC/N and k p = 42.2 %, respectively). Excess MnO2 inhibits the grain growth and degrades the ferroelectric and piezoelectric properties of the ceramics. Sintering temperature has an important influence on the microstructure, tetragonal–orthorhombic phase transition near 40 °C, ferroelectric and piezoelectric properties of the ceramics. The increase in sintering temperature leads to large grains and more noticeable tetragonal–orthorhombic phase transition near 40 °C, enhances ferroelectricity and thus improves effectively the piezoelectricity of the ceramics. The Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramic sintered at 1350 °C possesses the optimum piezoelectric constant d 33 value of 373 pC/N.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead-based piezoelectric ceramics with perovskite structure have been widely used in sensors, actuators as well as microelectronic devices due to their superior piezoelectric properties. However, the use of lead-based ceramics has caused serious environmental problems because of the high toxicity of lead oxide. Therefore, it is necessary to develop lead-free piezoelectric ceramics.
Barium titanate (BaTiO3) is a classic perovskite ferroelectric with tetragonal symmetry at room temperature. Zr4+- and Sn4+-modified BaTiO3 materials have been extensively investigated for dielectric applications [1, 2]. Compared with Pb(Zr,Ti)O3-based perovskite ceramics (d 33 = 289–710 pC/N) [3], pure BaTiO3 possesses relatively poor piezoelectricity (d 33 = 191 pC/N) [4]. Therefore, BaTiO3-based materials are widely used as dielectric materials but not piezoelectrics. However, recently, Liu and Ren [5] designed a ferroelectric system 0.5Ba(Zr0.2Ti0.8)O3–0.5(Ba0.7Ca0.3)TiO3 with a super high d 33 (~620 pC/N) and reported the origination of the high piezoelectricity from a TCP (tricritical point)-type MPB (morphotropic phase boundary). Since then, as one of promising candidates for lead-free piezoelectric materials, BaTiO3-based ceramics have attracted considerable attention. Many modified BaTiO3-based lead-free piezoelectric ceramics (e.g., (Ba1−x Ca x )(Ti0.98Zr0.02)O3 [6], (Ba0.95Ca0.05)(Ti1−x Zr x )O3 [7], ZnO-doped Ba0.85Ca0.15Ti0.90Zr0.10O3 [8], (Ba0.93Ca0.07)(Ti0.95Zr0.05)O3 [9], etc.) have been prepared and the effects of sintering temperature [10], dwell time during sintering [11], and poling conditions [12] on the piezoelectric properties of Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramics have been investigated. As known, for Pb(Zr,Ti)O3-based ceramics, the doping of metal oxides (e.g., La2O3, Nb2O5, MnO2, Fe2O3, etc.) is an effective approach to improve the electrical properties of the ceramics. Amongst the metal oxide dopants, MnO2 is the most interesting because of the multi-valence states (Mn2+, Mn3+, and Mn4+) of Mn ions [13, 14] and has been extensively used to substitute the A- or/and B-site ions of ferroelectrics for tailoring electrical properties[13–20]. It has been found that low level doping of MnO2 leads to “soft” and “hard” effects simultaneously and thus induce the increase in piezoelectricity (d 33 and k p) and relative permittivity ε r and the decrease in loss tangent tanδ in (Ca,Sr)Bi4Ti4O15- [13], PZT- [14], BNT- [19], and (Sr,Ca)NaNb5O15-based [20] ceramics. However, there is little work on MnO2 doping for 0.5Ba(Zr0.2Ti0.8)–0.5(Ba0.7Ca0.3)TiO3 (i.e., Ba0.85Ca0.15Ti0.90Zr0.10O3) ceramics. In this study, MnO2-doped Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramics were prepared by an ordinary sintering method and the effects of MnO2 on the microstructure, ferroelectric, and piezoelectric properties were studied. In addition, our previous study shows that the Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramic with large grains exhibits high piezoelectricity. Therefore, the possible relationship between electrical properties and grain size were briefly investigated by sintering the Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramic at different temperature.
Experimental
Ba0.85Ca0.15Ti0.90Zr0.10O3 + xmol% MnO2 (BCZT-Mn-x) ceramics were prepared by a conventional ceramic technology using metal oxides and carbonate powders: BaCO3(99 %, Sinopharm Chemical Reagent Co., Ltd, China), CaCO3(99 %, Sinopharm Chemical Reagent Co., Ltd, China), TiO2(98 %, Sinopharm Chemical Reagent Co., Ltd, China), ZrO2 (99 %, Sinopharm Chemical Reagent Co., Ltd, China), and MnO2(97.5 %, Sinopharm Chemical Reagent Co., Ltd, China). The powders in the stoichiometric ratio of Ba0.85Ca0.15Ti0.90Zr0.10O3 (BCTZ) were first mixed thoroughly in ethanol using zirconia balls for 10 h. After the calcination at 1100 °C for 4 h, MnO2 powder was added. The mixture was ball-milled again for 10 h, mixed thoroughly with a poly(vinyl alcohol) binder solution and then pressed into disk samples. After removal of the binder, the samples were sintered at 1250–1350 °C for 2 h. The sintered ceramics were coated with silver paste to form electrodes on both sides and fired at 810 °C for 10 min. The ceramics were poled at room temperature for 40 min in a silicone oil bath under a dc filed of 3 kV/mm.
The crystalline structure of the sintered samples was determined using X-ray diffraction (XRD) analysis with CuKα radiation (DX1000, China). The microstructures were observed using scanning electron microscopy (JSM-5900LV, Japan). The relative permittivity ε r at 1 kHz was measured as a function of temperature using an LCR meter (Agilent E4980A, USA). The polarization hysteresis (P–E) loops were measured using a ferroelectric measuring system (Premier II, Radiant Technologies, Inc., USA). The planar electromechanical coupling factor k p was determined by the resonance method according to the IEEE Standards 176 using an impedance analyzer (Agilent 4294A, USA). The loss tangent tanδ at 1 kHz was measured using an impedance analyzer (Agilent 4294A, USA). The piezoelectric constant d 33 was measured using a piezo-d 33 meter (ZJ-6A, China).
Results and discussions
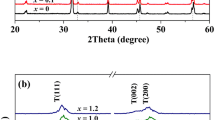
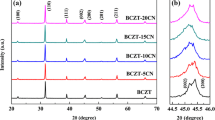
The XRD patterns of the BCZT-Mn-x ceramics are shown in Fig. 1. All the ceramics have a pure perovskite structure and no second phases were detected. The coexistence of orthorhombic and tetragonal phases near room temperature has been reported in Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramics [5]. The addition of MnO2 has no obvious influence on the crystal structure of the BCTZ ceramics.
The SEM micrographs of the BCZT-Mn-x ceramics with x = 0, 0.25, 0.75, and 2.0 are shown in Fig. 2. All the ceramics have a dense microstructure. From Fig. 2a, for the ceramic with x = 0, only a few large grains (about 15 μm) are distributed in large numbers of small grains (about 3 μm) (Fig. 2a). As x increases to 0.25, an inhomogeneous grain size distribution can be observed. The grains of the ceramic with x = 0.25 exhibit an obvious bimodal grain size distribution and the big grains with an average size of about 45 μm are surrounded by the small grains with an average size of 3.5 μm (Fig. 2b). However, as x further increases to 0.75 and 2.0, the grains become relatively uniform and much smaller. The average grain sizes for the ceramics with x = 0.75 and 2.0 are about 2.5 and 2.0 μm. Obviously, a small amount of MnO2 can promote effectively the grain growth, while excess MnO2 causes a significant inhibition of grain growth. Similar influence of MnO2 on microstructure has been observed in PZT-based ceramics [15, 16]. For the BCTZ-Mn-x ceramics, a small amount of MnO2 within the solubility limit of Mn ions can be dissolved in the perovskite structure and Mn ions substitute the B-site Ti4+ and Zr4+ of the ceramics to create oxygen vacancies. The oxygen vacancies increase the mobility of grain boundary and promote the mass transportation [15, 16]. As a result, the grain becomes large with x increasing. However, excess MnO2 may accumulate near the grain boundaries [14], reduce their mobility and thus suppress the grain growth [15, 16].
Figure 3a shows the P–E loops of BCTZ-Mn-x ceramics, while the variations of the remanent polarization P r and coercive field E c of the BCTZ-Mn-x ceramics with x are shown in Fig. 3b. All the ceramics exhibit a typical P–E loop for ferroelectrics (Fig. 3a). From Fig. 3b, the observed P r increases and then decrease with x increasing, giving the maximum value of 11.7 μC/cm2 at x = 0.25. For the ceramics with x ≥ 0.75, the observed P r has a weak dependence on x and retains the small values of about 3–4 μC/cm2. The observed E c keeps nearly unchanged (~0.32 kV/mm) with x increasing to 0.75 and then increases to 0.55 kV/mm with x further increasing to 2.0. The large P r in the ceramic with x = 0.25 may be attributed to the large grain size. Similar result has been observed in BaTiO3 ferroelectric [21].
Figure 4 shows the temperature dependence of ε r for the BCZT-Mn-x ceramics. All the BCTZ-Mn-x ceramics exhibit the paraelectric cubic-ferroelectric tetragonal phase transition peak at T C. The ferroelectric tetragonal–ferroelectric orthorhombic phase transition near 40 (T O–T) can be also observed, suggesting that orthorhombic and tetragonal phases coexist near room temperature in the BCTZ-Mn-x ceramics. As x increases from 0 to 0.25, the phase transition peaks at T O–T and T C becomes more noticeable. However, as x further increases, the transition peaks at T O–T and T C become gradually smeared. It has been found that for K0.5Na0.5NbO3 [22], BaTiO3 [23], and PbTiO3 [24] ceramics, fine grain weakens the dielectric peak’s sharpness and intensity and smears phase transition. Similarly, the weakness of the phase transitions at T O–T and T C of the BCTZ-Mn-x ceramics with x ≥ 0.5 should be attributed to the small grain size as shown in Fig. 2c, d.
The variations of d 33, k p, ε r, and tanδ with x for the BCTZ-Mn-x ceramics are shown in Fig. 5. The observed d 33, k p, and ε r increase with x increasing and then decrease, giving the maximum values of 306 pC/N, 42.0 % and 3427 at x = 0.25, respectively. When x ≥ 0.75, the observed d 33, k p, and ε r exhibit relatively weak dependence on x. The observed tanδ decreases with increasing x and then increases, reaching a minimum value of 1.0 % at x = 0.75. It is known that a small amount of MnO2 induces the combinatory effects of donor and acceptor doping in PZT-, Bi0.5Na0.5TiO3−, and (Ca1−x Sr x )Bi4Ti4O15-based ceramics [14–16]. At high temperature, Mn2+ (0.091 nm) and Mn3+ (0.07 nm) coexist [13, 14]. For the BCTZ-Mn-x ceramics with x ≤ 0.25, Mn2+ and Mn3+ can enter the A-sites (Mn3+: donor doping), causing the increase in the d 33, k p, and ε r. Simultaneously, part of Mn3+ ions enter the B-sites (acceptor doping), leading to the decrease in tanδ. As x increase above 0.25 mol %, the solubility limit of Mn ions in the A- and B-sites of the ceramics is reached. Excess Mn ions may accumulate near the grain boundaries and thus lead to the degradation of the d 33, k p, ε r, and tanδ.
From Figs. 2, 3, 4, 5, the BCTZ-Mn-0.25 ceramic with large grains possesses stronger ferroelectricity, more noticeable phase transition peaks at T C and T O–T and better piezoelectricity than the ceramics with small grains. Therefore, it may be inferred that the grain size of BCTZ-Mn-x ceramics has an important influence on the phase transition and electrical properties. To demonstrate the effects of grain size on phase transition and electrical properties of the BCTZ-Mn-x ceramics, a well-known 0.5Ba(Zr0.2Ti0.8)–0.5(Ba0.7Ca0.3)TiO3 composition [5] (i.e., BCTZ, the BCTZ-Mn-0 ceramics without MnO2 doping) was selected and sintered at different temperature. Figure 6 shows the SEM micrographs of the BCTZ ceramics at different sintering temperature T s. The ceramic sintered at 1250 °C has a small average grain size of 3.8 μm (Fig. 6a). The evolution process of grain growth with T s can be clearly observed: as T s increases from 1250 to 1275 °C, the grains become slightly large and denser; when T s increases to 1300 °C, a few large grains began to appear; with T s increasing to 1325 °C, part of the grains began to growth up and the grains have a bimodal grain size distribution with large grains of diameters about 48 μm uniformly distributed among the grains which are of much smaller diameters, about 3.7 μm; and as T s further increases to 1350 °C, the rest of small grains grow into large grains. The average grain sizes of the ceramics sintered at 1275, 1300, 1325, and 1350 °C for 2 h are about 4.2, 5.2, 28.4, and 33.0 μm, respectively.
The temperature dependences of ε r for the BCTZ ceramics sintered at different T s are shown in Fig. 7. As grain size increases, the phase transition peaks near 90 °C (T C) becomes gradually sharper. It is also observed that the phase transition near 40 °C (T O–T) gradually appears and becomes more noticeable. Obviously, the grain size has a significant influence on the phase transition characteristics of the BCTZ ceramics. Similar phenomena have been observed in K0.5Na0.5NbO3, BaTiO3, and PbTiO3 ceramics [22–24].
The P–E loops of BCTZ ceramics sintered at different T s are shown in Fig. 8a, while the variations of P r and E c of the BCTZ ceramics with T s are shown in Fig. 8b. For the ceramic sintered at 1250 °C, a flattened and slanted loop is observed. As T s (grain size) increases to 1350 °C, the loop becomes gradually saturated and square like. At T s = 1350 °C, a large P r (10.1 μC/cm2) and a relatively low E c (0.22 kV/mm) are obtained (Fig. 8a). From Fig. 8b, the observe P r increases with T s increasing, while the observed E C decreases with T s increasing. It can be noted that the ceramics with large grains exhibits larger P r and lower E C than the ceramics with small grains. This should be attributed to the easier polarization reversal process of ferroelectric domain in larger grains than in smaller grains [17, 21].
Figure 9 shows the variations of d 33, k p, ε r, tanδ, and grain size with T s for the BCTZ ceramics. For the BCTZ ceramics sintered at T s < 1300 °C, the observed d 33 and k p exhibit a weak dependence on T s and give the relatively low values of about 30 pC/N and 11.0 %, respectively. However, as T s further increases to 1350 °C, the observed d 33 and k p increase greatly to 373 pC/N and 53.0 %. The observed ε r increases monotonously from 2159 to 3974 with T s increasing from 1250 to 1350 °C. The observed tanδ increases and then decreases with T s increasing, reaching a maximum value of 2.98 % at T s = 1300.
It can be seen from Figs. 2, 3, 4, 5, 6, 7, 8, and 9 that grain size in the BCTZ ceramic is positively related with the coexistence of orthorhombic and tetragonal phases, ferroelectricity and piezoelectricity of the BCTZ ceramic; and the BCTZ ceramics with large grains possess much better ferroelectricity and piezoelectricity than those with small grains. It has been known that grain size has an important influence on the electrical properties of piezoelectric ceramics [22, 25–27]. From Figs. 2, 3, 4, 5, 6, 7, 8, and 9, well-saturated and square like P–E loops with large P r are observed and good piezoelectricity is obtained in the ceramics with large grains, while the ceramics with small grains exhibit slanted and narrowed loops with small P r and possess poor piezoelectricity. The internal mechanisms of the strong dependence of ferroelectric and piezoelectric properties on grain size may be attributed to the variation of domain structure with grain size [28–30]. In general, the correlation between gain size and domain size can be expressed [28, 29] as (Domain size) ∝ (grain size)m, where m is related to the grain size. As grain size increases, domain size increases and domain boundary decreases [15]. This leads to the increase in the contribution to polarization switching and piezoelectric response from extrinsic effect (domain wall motion) [17] and thus larger polarization is obtained in the ceramics with large grains than in those with small grains, which causes the significant improvement in the piezoelectric and ferroelectric properties in the ceramics with large grains. Compared with the ceramics with large grains, the motion of domain walls in the ceramics with small grains is more difficult [30]. Therefore, it can be concluded that the increase in the domain size leads to a significant improvement in the ferroelectricity and piezoelectricity in the ceramics with large grains. On the other hand, according to the thermodynamic theory of ferroelectrics, the piezoelectric coefficient d 33 can generally be expressed as a function of its relative permittivity ε r and spontaneous polarization P s [31]: d 33 = 2Q 11 ε 0 ε r P s, where Q 11 is the electrostrictive coefficient which is related to the domain structure and should not change significantly for perovskite materials. Accordingly, d 33 is greatly dependent on εr and P s. From Figs. 3, 5, 8, and 9, the ceramics with large grains possesses relatively large P s and ε r and thus exhibits the highest d 33. It should be also noted that the ceramics with large grains exhibit more noticeable tetragonal–orthorhombic phase transition than those with small grains. This clearer coexistence of tetragonal and orthorhombic phase also enhances the piezoelectricity of the ceramics with large grains. The effects of grain size on electrical properties and phase transitions in the BCTZ will be further studied systematically.
Conclusions
Lead-free ceramics Ba0.85Ca0.15Ti0.90Zr0.10O3 + xmol % MnO2 were prepared by an ordinary sintering technique and the effects of MnO2 and sintering temperature on microstructure, phase transition, and electrical properties of Ba0.85Ca0.15Ti0.90Zr0.10O3 lead-free ceramics were studied. A small amount of MnO2 (x ≤ 0.25) promotes the grain growth, improves the ferroelectricity and strengthens the tetragonal-orthorhombic phase transition near 40 °C. However, excess MnO2 has an inhibition of grain growth, weakens the phase transition near 40 °C and thus degrades the ferroelectric and piezoelectric properties of the ceramics. Because of the coexistence of tetragonal and orthorhombic phases near room temperature and the combinatory effects of soft and hard doping of Mn ions, the d 33, k p, and ε r are enhanced considerably after 0.25 mol % MnO2 doping. The ceramic with x = 0.25 exhibits the optimum piezoelectric properties (d 33 = 306 pC/N and k p = 42.2 %, respectively). The sintering temperature has an important influence on microstructure, tetragonal-orthorhombic phase transition near 40 °C and electrical properties of the Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramics. The grain size is positively related with ferroelectric and piezoelectric properties. The ceramics with large grains possess more noticeable tetragonal–orthorhombic phase transition, stronger ferroelectricity and thus exhibits better piezoelectric properties than the ceramics with small grains. The Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramic sintered at 1350 °C possesses the high d 33 value of 373 pC/N. The electrical properties and phase transition characters of the Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramics are very sensitive to sintering temperature and grain size.
References
Kuang SJ, Tang XG, Li LY, Jiang YP, Liu QX (2009) Scr Mater 61:68
Wei X, Wan X, Xi Y (2008) J Electroceram 21:226
Haertiling GH (1999) J Am Ceram Soc 82:797
Bechmann R (1956) J Acoust Soc Am 28:347
Liu W, Ren X (2009) Phys Rev Lett 103:257602
Li W, Xu Z, Chu R, Fu P, Zang G (2010) Mater Lett 64:2325
Zhang SW, Zhang HL, Zhang BP, Yang S (2010) J. Alloys Compd 506:131
Wu J, Xiao D, Wu W, Chen Q, Zhu J, Yang Z, Wang J (2011) Scr Mater 65:771
Li W, Xu Z, Chu R, Fu P, Zang G (2011) Mater Sci Eng, B 176:65
Wang P, Li Y, Lu Y (2011) J Eur Ceram Soc 31:2005
Wu J, Xiao D, Wu W, Zhu J, Wang J (2011) J Alloys Compd 509:L359
Su S, R. Zuo Z, Lu SB, Xu ZK, Wang XH, Li LT (2011) Curr Appl Phys 11:120–121
Li G, Zheng L, Yin Q (2005) J Appl Phys 98:064108
Hou Y, Zhu M, Gao F, Wang H, Wang B, Yan H, Tian C (2004) J Am Ceram Soc 87:847
Yu CS, Hsieh HL (2005) J Eur Ceram Soc 25:2425
Li SM, Lee SH, Yoon CB, Kim HE, Lee KW (2007) J Electroceram 18:311
Yan Y, Cho KH, Priya S (2011) J Am Ceram Soc 94:3953
Mgbemere HE, Herber RP, Schneider GA (2009) J Eur Ceram Soc 29:1729
Nagata H, Takenaka T (2001) J Eur Ceram Soc 21:1299
Fan X, Wang Y, Jiang Y (2011) J Alloys Compd 509:6652
Qiao L, Bi X (2009) J Eur Ceram Soc 29:1995
Buixaderas E, Bovtun V, Kempa M, Savinov M, Nuzhnyy D, Kadlec F, Vaněk P, Petzelt J, Eriksson M, Shen Z (2010) J Appl Phys 107:014111
Park Y, Lee WJ, Kim HG (1997) J Phys: Condens Matter 9:9445
Chattopadhyay S, Ayyub P, Palkar VR, Multani M (1995) Phys Rev B 52:13177
Damjanovic D, Demartin M (1997) J Phys: Condens Matter 9:4943
Zhang L, Zhong WL, Wang CL, Zhang PL, Wang YG (1998) Phys Stat Sol (a) 168:543
Martirenat HT, Burfoot JC (1974) J Phys C: Solid State Phys 7:1974
Cao W, Randall CA (1996) J Phys Chem Solids 57:1505
Randall CA, Kim N, Kucera JP, Cao W, Shrout TR (1998) J Am Ceram Soc 81:677
Demartin M, Damjanovic D (1996) Appl Phys Lett 68:3046
Damjanovic D (1998) Rep Prog Phys 61:1267
Acknowledgements
This study was supported by the projects of Education Department of Sichuan Province (11ZA104), Science and Technology Bureau of Sichuan Province (2010JQ0046) and the Open Projects of State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials of Southwest University of Science and Technology (10zxfk27) and State Key Laboratory of Electronic Thin Films and Integrated Devices of University of Electronic Science and Technology of China (KFJJ201108).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jiang, M., Lin, Q., Lin, D. et al. Effects of MnO2 and sintering temperature on microstructure, ferroelectric, and piezoelectric properties of Ba0.85Ca0.15Ti0.90Zr0.10O3 lead-free ceramics. J Mater Sci 48, 1035–1041 (2013). https://doi.org/10.1007/s10853-012-6835-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6835-y