Abstract
La1−x Sr x FeO3 (x = 0.0–1.0) nanoparticles have been synthesized by a sol–gel method. The samples are characterized by thermogravimetric/differential thermal analysis (TG/DTA), X-ray diffraction (XRD), fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The La1−x Sr x FeO3 (x = 0.0–1.0) materials have been sintered at 650 °C. Both the structural properties and phase transitions with increasing strontium content in La1−x Sr x FeO3 are presented by analysis of XRD data. Formaldehyde gas-sensing properties of La1−x Sr x FeO3 have been investigated. The experimental results show that the optimum operating temperatures of La1−x Sr x FeO3 varies with Sr content of x. Among all the samples, La0.7Sr0.3FeO3 shows a maximum response to formaldehyde. Finally, the formaldehyde gas-sensing mechanism has been presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Formaldehyde (HCHO), a colorless, strong-smelling gas, is one of the harmful indoor volatile organic compounds (VOCs). It, a possible carcinogen [1], can cause nervous system damage [2], immune system disorders [3], and respiratory disease [4]. The increasing concern over HCHO monitoring and control environmental pollution has generated great interest in developing new sensing materials and sensor technology in the last decade. The focus is not only on the development of new sensing materials to improve sensitivity, selectivity, and stability of sensors, but also on the development of new and better fabrication techniques to ensure reliability, safety, reproducibility, and low cost. Compared with the techniques to detect indoor HCHO [5–8], semiconductor gas sensor has been investigated extensively due to low cost, simple structure, as well as simple readout and sensor operation. Semiconductor materials for detecting HCHO include NiO [9], ZnO/ZnSnO3 [10], CdO-In2O3 [11], NiO-doped SnO2 [12], Ni-doped ZnO [13], MWCNTs (multi-wall carbon nanotubes)-doped SnO2 [14], and La–Bi–Fe–O [15].
It is reported that synthetic methods have great influence on the structural and electrical properties of semiconductor oxides [16, 17]. Therefore, numerous methods have been investigated to prepare semiconductor oxides such as electrochemical synthesis [18], co-precipitation [19], solid-state reaction [20], combustion [21], hydrothermal process [22], citric-EDTA complexation [23], and sol–gel process [24]. Compared with other methods, the sol–gel process has many advantages including excellent chemical stoichiometry, lower operating temperature, convenience for controlling the process and nanoscale particles.
Perovskite-type oxides with the general formula ABO3 (A: rare earth, B: transition metal) and A1−x A′ x BO3 or AB1−x B′ x O3 (A-site or B-site substituted ABO3) have been widely studied in the fields of catalysts [25], fuel cells [26], and oxygen transport membranes [27]. The perovskite-type oxides have attracted much attention on gas-sensing properties due to their stability and sensitivity [28, 29]. LaFeO3 can be used as a sensing material for detecting humidity, CO, and ethanol vapor [30–32]. La-site or Fe-site-substituted LaFeO3 has been used as ethanol or formaldehyde gas-sensing materials [33–36]. It is found that the gas-sensing behaviors vary with composition x in A1−x A′ x BO3 or AB1−x B′ x O3 materials [37, 38].
In this study, strontium-substituted LaFeO3 with the general formula of La1−x Sr x FeO3 (x = 0.0–1.0) was prepared by a sol–gel technique. The structural characteristics of La1−x Sr x FeO3 were investigated using various techniques including TG/DTA, XRD, FTIR, and TEM. The HCHO sensing properties of all samples were carried out at gas concentration ranging from 10 to 100 ppm to select a suitable composition material with a low operating temperature and high sensitivity sensing. Thus, these oxides were examined as novel sensing materials to HCHO.
Experimental
Preparation of La1−x Sr x FeO3 materials
La1−x Sr x FeO3 (x = 0.0–1.0) materials were prepared by a sol–gel method with analytical grade La(NO3)3·6H2O, Sr(NO3)2, Fe(NO3)3·9H2O, and citric acid as starting materials. 18.077 g citric acid was dissolved in 25 ml deionized water to yield [citric acid] = 3.44 M and was heated to 80 °C with continuous stirring. Stoichiometric quantities of La(NO3)3·6H2O, Sr(NO3)2, and Fe(NO3)3·9H2O were dissolved in 25 ml deionized water to yield [Fe] = 0.344 M. Then the nitrate salts solution was added dropwise into citric acid solution to make a homogeneous mixture. The mixed aqueous solution was heated with continuous stirring for about 6 h until a highly viscous gel formed. The gel was dried at 120 °C for 24 h to get dry foamy powders, followed by grinding to form a fine precursor. Finally, the precursor was calcined at 650 °C for 6 h.
Characterization of La1−x Sr x FeO3 materials
X-ray diffraction (XRD) patterns of the powders were examined in 2θ region of 20–80° with a scanning speed of 4° min−1 on Rigaku, Model D/MAX 2400, Japan, with Cu Kα (1.5406Å) radiation. The thermal decomposition behavior of the precursor powders was recorded by means of thermogravimetry (TG) and differential thermal analysis (DTA) (TGA/SDTA851e, Mettler-Toledo, Switzerland). A heating rate of 10 °C min−1 was used for the measurements from room temperature to 800 °C. Fourier transform infrared spectroscopy (FTIR) spectra were measured as KBr disks using a Bruker EQUINOX 55 with a spectral range from 2000 cm−1 to 400 cm−1. Chemical analysis was performed using a SRS 3400 X-ray fluorescence spectrometer. Transmission electron microscopy (TEM) was carried out to obtain direct information about the size and structure by Tecnai G220 S-Twin transmission electron microscope. The grains were dispersed in ethanol on a carbon-coated TEM copper grid.
Gas sensors fabrication and measurements
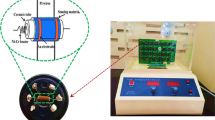
The as-prepared samples were mixed with deionized water and carefully ground into paste in an agate mortar. The paste was coated onto a hollow ceramic tube (Ø 1 mm × 4 mm) with two electrodes spaced 1 mm, and subsequently sintered at 600 °C for 2 h. A heater consisting of a 30 Ω resistor wire was inserted through the tube. The power consumption of the heater is 0.3 W at the working temperature of 200 °C. The electrodes and heater wires were soldered on a base element [14].
The gas-sensing properties of the gas sensor were processed in a sealed chamber of an automatic testing system. The volume of the chamber was 1200 cm3. Figure 1 gives a sketch diagram of the measuring system [39]. Bottled formaldehyde gas with concentration of 100 ppm balanced by nitrogen vapor was used. The carrier gas was bottled synthetic clean air. Both the bottled formaldehyde gas and the bottled air were produced by Guangming Special Vapors Institute of Dalian, China. The formaldehyde gas and the air were mixed in certain ratios using two mass flow controllers (MFCs) of 200 sccm (standard cubic centimeter per minute) for both formaldehyde and air. The dry mixed gas was sent into a sealed testing chamber. For a target concentration, the flow ratio was calculated as follows:
where C HCHO is the target HCHO concentration (ppm). C BottledHCHO is the concentration of bottled formaldehyde gas (ppm), V 1 and V 2 are two MFCs’ controlling voltages (V). F 1 and F 2 are two MFCs’ maximum flow (sccm). The controlling voltage was 5 V when MFC reached its highest value. C BottledHCHO = 100 ppm and F 1 = F 2 = 200 sccm in this case. The procedure was as follows: pure air was introduced into the sealed chamber first. When the voltage of the sensor was stable, the detected gases were introduced into the testing chamber. Finally, the pure air was introduced for cleaning the testing chamber after the voltage of the sensor was steady in the detected gases.
The MFCs were operated by output signals of a D/A data acquisition card controlled by a computer. The change of the voltage of the sensor indicated the change of the conductivity of the sensor surface, which was caused by the change of gas concentration in the chamber. A computer monitored and recorded the change of the voltage signal by an A/D data acquisition card.
The working temperature of the sensor was provided by applying a DC power (GPS-3303C, Guwei Electronic, Taiwan) to the heater. The working temperature range of the sensor was from 160 to 440 °C. In order to improve stability and repeatability, the sensor was aged at 300 °C in air for 240 h. The sensor was connected with an external resistor in series at a voltage of 5 V. The resistance of the gas sensor in target gas is calculated as the follows: R S = R L × (5 − V L)/V L, where R S, R L, and V L are the resistance of the sensor, the resistance of the reference resistor, and the measured voltage, respectively. Sensor response is defined as S = R g /R a , where R g and R a are electrical resistance in testing gas and in air, respectively.
Results and discussion
TG/DTA analysis
Figure 2 presents thermal decomposition curves of La0.8Sr0.2FeO3 precursor. TG curve changes slowly below 150 °C, which may be regarded as a result of evaporation of residual water in the dried precursor powders. From 150 to 460 °C, the weight loss is about 78 %, which is accompanied by two remarkable exothermic peaks around 250 °C and 360 °C depicted in DTA curve. The first exothermic reaction, between 150 and 305 °C, is mainly due to the decomposition of the organic compound. The second one, from 305 to 460 °C, is attributed to the process of formation of perovskite crystalline structure. No further weight loss or peak appears beyond 500 °C, revealing that all the organic materials have been burnt out and there is no change in the phase of La0.8Sr0.2FeO3. The reaction mechanism can be formulated in terms of equation as follows:
Structural properties
According to the thermal analysis of TG/DTA, 400, 500, 600, and 650 °C were selected to sinter the La0.8Sr0.2FeO3 precursor. Figure 3 displays XRD patterns of La0.8Sr0.2FeO3 sintered at different temperatures for 6 h. It can be seen from Fig. 3a that three broad band peaks appear at around 32°, 46°, and 57° of 2θ, respectively, which reveals that an elementary perovskite phase is formed. The major perovskite peaks (JCPDS 35-1480) are all presented in the XRD patterns of 500, 600, and 650 °C (shown in Fig. 3b–d), but minor phases of SrCO3 (JCPDS 05-0418) are also found in the patterns.
The FTIR spectra of La0.8Sr0.2FeO3 precursor sintered at different temperature in the frequency range of 2000–400 cm−1 are shown in Fig. 4. The spectra display absorption bands at 594, 575, 573, and 573 cm−1 for the materials sintered at 400, 500, 600, and 650 °C, respectively. These bands are related to Fe–O stretching vibration located in the octahedral site [40, 41]. It can be inferred that the elementary perovskite phase is formed after the material was annealed at 400 °C for 6 h. Small absorption bands centered at 474, 467, and 474 cm−1 are shown in Fig. 4b–d, respectively. These small bands belong to the stretching vibrations of Sr2+ situated at 12 coordinated position [42]. With the increase of sintering temperature, the compound formation is enhanced, which can be confirmed by the adsorption bands shifting from higher frequency to lower frequency. The FTIR peaks at around 1640 cm−1 are attributed to the stretching vibration of O–H of adsorbed water molecules from the ambient [41]. The FTIR peaks related to SrO appear at 1072, 1068, and 1066 cm−1 in Fig. 4a–c, respectively, but it becomes almost flat in Fig. 4d. As shown in Fig. 4a-d, the absorption bands appearing at 1460 cm−1 and 860 cm−1 are attributed to the asymmetry vibration and out-of-plane bending vibration of CO −23 [43]. The content of SrCO3 decreases as the sintering temperature increases. The results are confirmed by the appearance of SrCO3 displayed in Fig. 3. These may be due to the decomposition of the organic compound during sintering process, a large number of CO2 is released immediately, which leads to the formation of SrCO3 with SrO decomposed from Sr(NO3)2. This phenomenon was also observed by J. Karpinski et al. [44, 45]. Most of the alkaline earth metal oxides and rare earth metal oxides, in particular BaO, SrO, and Re2O3, contain metal carbonate [46, 47].
The semi-quantitative elemental composition of the as-prepared La0.8Sr0.2FeO3 was experimentally determined using X-ray fluorescence spectrometer. The obtained experimental stoichiometric ratio of La:Sr:Fe:O is 0.795:0.218:1:2.904, which matches the expected composition based on synthesis quantities. The partial substitutions at La-site with Sr create structural defects such as oxygen vacancies, which may greatly affect gas-sensing properties. Oxygen vacancies can act as preferential adsorption sites for gas molecules. The surface defects often dominate the electronic/chemical properties and adsorption behaviors of semiconductor oxide surfaces. Oxygen vacancies bind more tightly with gas molecules attracting more charges from the surfaces compared with oxygen-vacancy-free surface [48].
Figure 5 shows the XRD patterns of La1−x Sr x FeO3 (x = 0.0–1.0) samples sintered at 650 °C for 6 h. The main peaks of La1−x Sr x FeO3 (x = 0.0) are identified as LaFeO3 (JCPDS 75-0541) perovskite, and the main phase in La1−x Sr x FeO3 (x = 1.0) is confirmed as SrFeO2.97 (JCPDS card 40-0905). All the XRD patterns of La1−x Sr x FeO3 (x = 0.1–0.9) are similar to those of LaFeO3 or SrFeO2.97, but some impurity phases related to SrCO3 (JCPDS card 05-0418) are seen in La1−x Sr x FeO3 (x = 0.3, 0.4, 0.6–1.0), and some impurity peaks related to Fe3O4 (JCPDS 28-0491) and La2O3 (JCPDS 24-0554) are shown in La1−x Sr x FeO3 (x = 0.0 and 0.1). However, no impurity phase is found in La1−x Sr x FeO3 (x = 0.4 and 0.5). Table 1 presents the XRD parameters, such as lattice constant, cell volume, the full width at half maximum (FWHM), crystallite size, space group, and symmetry, calculated from the XRD patterns. The lattice parameters are calculated by the d = (h 2/a 2 + k 2/b 2 + l 2/c 2)−1/2) formula from the XRD patterns. In addition, the crystallite size is evaluated by Debye Scherror formula of 0.9λ/βcosθ, where λ is the wavelength of the target CuKα 1.5406 Å and β is the full width at half maximum of 32° in 2θ. The crystallite size increases with the increasing of the content of strontium except in the cases of x = 0.7 and the end members. Similarly, as the content of strontium increases, diffraction peaks of La1−x Sr x FeO3 perovskite phase shift to higher 2θ direction, and they become correspondingly narrow. The unit cell volume decreases with the increasing of Sr content in La1−x Sr x FeO3 (x = 0.1–0.9), indicating that the replacement of La3+ by Sr2+ leads to lattice contraction. Although the ionic radius of Sr2+ is larger than that of La3+, the replacement of La3+ by Sr2+ in La1−x Sr x FeO3 may lead to an increased covalency between the iron and oxygen ions and a decreased bond length of Fe–O due to the formation of Fe4+ ions, which reduces the unit cell volume [49]. Phase transitions with increasing strontium content in La1−x Sr x FeO3 are displayed (see Table 1). The phase is cubic (space group Pm-3 m) for LaFeO3. For x = 0.1–0.3, the XRD patterns are refined using an orthorhombic phase in the space group Pnma. A rhombohedral phase (space group R-3c) is observed for x = 0.4–0.9. A phase transition to cubic (Pm-3 m) occurs when x = 1.0.
The SEM micrographs of La0.7Sr0.3FeO3 and La0.8Sr0.2FeO3 powder sintered at 650 °C are shown in Fig. 6. These micrographs indicate agglomerated and well-defined particles with homogeneous crystallite size distribution. The porous feature of the samples may be attributed to the liberation of a large amount of gas during sintering process.
Figure 7 shows the TEM image of La0.7Sr0.3FeO3 sample with average particle size of 20–25 nm, which is consistent with the crystallite size calculated by Debye Scherror formula of 0.9λ/βcosθ in Table 1.
Formaldehyde gas-sensing properties
Gas-sensing experiments were performed at different operating temperatures to find the optimum operating condition. Figure 8 displays the relationship between the different operating temperature and the response of La1−x Sr x FeO3 (x = 0.0–1.0) sensors to 50 ppm HCHO. The optimum operating temperatures and the response of La1−x Sr x FeO3 gas sensors are listed in Table 2. It can be seen that the optimum temperatures vary with x. Among the sensors, La0.7Sr0.3FeO3 sensor shows the maximum response of about 26 to 50 ppm HCHO at the optimum operating temperature of 200 °C.
The responses of La1−x Sr x FeO3 sensors to different HCHO concentrations at their own optimum temperatures are shown in Fig. 9. According to the relationship between the responses of the sensors and strontium content x, it can be easily seen that the sensors response curves are separated into four sets A, B, C, and D. For the sensors in sets A-C, the response of the sensors in set A is higher than those of the sensors in sets B and C, and the response of the sensors in set C is the lowest. The responses of the sensors in set A decrease with x increasing from 0.3 to 0.6 when the sensors are exposed to 10–50 ppm HCHO. For the sensors set B, the responses decrease with x varying from 0.0 to 0.2. For the sensors in set C, the response of La0.2Sr0.8FeO3 sensor is larger than that of La0.3Sr0.7FeO3 sensor. All the sensors in sets A-C show a good linear relationship between the responses and HCHO concentrations. However, the sensors in set D increase rapidly from 30 to 40 ppm HCHO. The responses of the sensors in sets A-C decrease with x varying with 0.3, 0.4, 0.5, 0.6, 0.0, 0.1, 0.2, 0.8, and 0.7. Figure 9b shows the responses of La0.7Sr0.3FeO3 sensors to 10–100 ppm HCHO concentrations at 200 °C. It is obviously found that the La0.7Sr0.3FeO3 sensor shows the highest response to HCHO among all the La1−x Sr x FeO3 (x = 0.0–1.0) sensors. The high difference of La1−x Sr x FeO3 sensors responses may be mainly due to the different compositions of the materials. The gas-sensing properties are relative to crystallite size, surface state, oxygen adsorption quantity, activation energy of oxygen adsorption, and lattice defects. These parameters depend on the changing composition of materials [50]. For example, with an increase in amount of Sr dopant, the optimal operating temperature of La x Sr1−x Fe x O3 sensors varies, which implies that Sr doping may have an effect on the adsorption activation energy of the material.
Figure 10 illustrates the response and recovery time of La0.7Sr0.3FeO3 gas sensor in air and in 50 ppm HCHO. The response and recovery times (defined as the time required to attain 90 % of the final value) of the sensor to 50 ppm formaldehyde are about 260 s and 70 s, respectively. The response and recover times are much longer than those of previously published (between 10 s and 60 s for different concentrations of formaldehyde [10, 12, 13, 15, 35, and 36]). This delay is due to a slow gas exchange in the test chamber concerning the volume of the chamber and the gas flow rate.
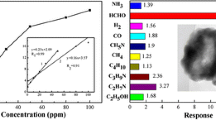
In order to study the selectivity of the La0.7Sr0.3FeO3 gas sensor to formaldehyde, the responses of the sensor to 50 ppm acetone, ammonia, methanol, benzene, ethanol, and toluene at 200 °C were carried out. As is shown in Fig. 11, the response of the sensor to formaldehyde is higher than the responses of the sensor to other gases. The response of the sensor to formaldehyde is 26, while to acetone, ammonia, methanol, benzene, ethanol, and toluene, the responses are 9, 1.2, 10.5, 1.4, 19, and 1.5, respectively, which indicates that ethanol is the main interference gases for the sensor. Cross sensitivity is a well-known disadvantage for the metal oxide gas sensors. The selectivity of La0.7Sr0.3FeO3 gas sensor can be improved by optimizing the doping (elements and their concentration) [51], the operation temperature [52], or neural network methods [53]. The detailed information will be discussed in future.
In order to investigate the long time stability of the La0.7Sr0.3FeO3 gas sensors, the responses of the two sensors to 50 ppm formaldehyde were repeated every 5 days for total 30 days. Figure 12 gives the responses of two La0.7Sr0.3FeO3 gas sensors to 50 ppm formaldehyde at 200 °C. It can be seen from the figure that each sensor exhibits a nearly constant sensor signal during the test, indicating a good stability of the La0.7Sr0.3FeO3 gas sensors. The inset in Fig. 12 shows the transient response of the sensors to 50 ppm formaldehyde at 200 °C. The reversibility and repeatability of two La0.7Sr0.3FeO3 gas sensors are also satisfactory.
Gas-sensing mechanism
The resistance increases when the sensors are exposed in formaldehyde, which reveals that La1−x Sr x FeO (x = 0.0–1.0) shows p-type semiconductor conductivity behavior in formaldehyde gas. The possible formaldehyde gas-sensing mechanism is suggested as follows:
The La1−x Sr x FeO3-based sensor is influenced by the oxygen adsorbed on the surface of the material. The metal ions on the surface of La1−x Sr x FeO3 can adsorb atmospheric oxygen easily. The electronic states of the adsorbed oxygen species on the surface of La1−x Sr x FeO3 materials undergo following reactions [54]:
where the subscripts gas and ads represent molecular oxygen and the state of adsorption, respectively. When temperature increases, the equilibrium shifts to the right. This will decrease the barrier height for a hole to transport and the oxygen species capture electrons from the sensing materials, which will lead to the decreasing of the resistance. Since adsorption is an exothermic reaction, the quantity of adsorbate decreases at higher temperature. However, since the oxidation of formaldehyde is thermally activated, the reaction rate increases depending on temperature. The response results from the interaction of the above two reactions, thus it reached to an optimum [55].
When in the presence of reducing gas HCHO, the oxygen adsorbed on the surface of the sensing material react with HCHO as follows [39, 56]:
The products could be formic acid (CHOOH) and/or water, and CO2. Both of these reactions release electrons. The released electrons are compensated with the holes of La1−x Sr x FeO3, which leads to the decreasing of conductivity of the material. As a result, the resistance of the sensor will increase.
Conclusions
Nanocrystalline La1−x Sr x FeO3 (x = 0.0–1.0) powders have been synthesized by a sol–gel method. It is found that the main phases of all the samples sintered at 650 °C are perovskite structures. Some impurity phases related to SrCO3, Fe3O4, and La2O3 are shown in La1−x Sr x FeO3 (x = 0.0–0.3, 0.6–1.0). However, no impurity phase is found in La1−x Sr x FeO3 (x = 0.4, 0.5). As Sr content of x increases, the phase varies with cubic, orthorhombic, and rhombohedral and cubic phase. XRD data and TEM images confirm that the average crystallite size of La0.7Sr0.3FeO3 is about 25 nm. The La1−x Sr x FeO3 materials present a p-type semiconductor behavior in formaldehyde. The optimum working temperatures of La1−x Sr x FeO3 change with composition x, and the responses of La1−x Sr x FeO3 to HCHO depend on composition x. Among La1−x Sr x FeO3 (x = 0.0–1.0) materials, La0.7Sr0.3FeO3 shows the maximum response of about 26 to 50 ppm HCHO at the optimum operating temperature of 200 °C. The response and recovery times of La0.7Sr0.3FeO3 to 50 ppm HCHO are 260 s and 70 s, respectively. Ethanol is the main interference gases for the La0.7Sr0.3FeO3 sensor.
References
Soffritti M, Maltoni C, Maffei F, Biagi R (1989) Toxicol Ind Health 5:699. doi:10.1177/074823378900500510
Songur A, Ozen OA, Sarsilmaz M (2010) Rev Environ Contam Toxicol 203:105. doi:10.1007/978-1-4419-1352-4_3
Thrasher JD, Broughton A, Madison R (1990) Arch Environ Health 45:217. doi:10.1080/00039896.1990.9940805
Subramaniam RP, Chen C, Crump KS, DeVoney D, Fox JF, Portier CJ, Schlosser PM, Thompson CM, White P (2008) Risk Anal 28:907. doi:10.1111/j.1539-6924.2008.01083.x
Kuijpers ATJM, Neele J (1983) Anal Chem 55:390. doi:10.1021/ac00253a052
Hunter MC, Bartle KD, Seakins PW, Lewis AC (1999) Anal Commun 36:101. doi:10.1039/A809762C
Septon JC, Ku JC (1982) Am Ind Hyg Assoc J 43:845. doi:10.1080/15298668291410675
Kim S, Kim HJ (2005) Bioresour Technol 96:1457. doi:10.1016/j.biortech.2004.12.003
Dirksen JA, Duval K, Ring TA (2001) Sens Actuators B 80:106. doi:10.1016/S0925-4005(01)00898-X
Xu JQ, Jia XH, Lou XD, Xi GX, Han JJ, Gao QH (2007) Sens Actuators B 120:694. doi:10.1016/j.snb.2006.03.033
Chen T, Liu QJ, Zhou ZL, Wang YD (2008) Sens Actuators B 131:301. doi:10.1016/j.snb.2007.11.025
Lv P, Tang ZA, Yu J, Zhang FT, Wei GF, Huang ZX, Hu Y (2008) Sens Actuators B 132:74. doi:10.1016/j.snb.2008.01.018
Bai ZK, Xie CS, Hu ML, Zhang SP (2008) Physica E 41:235. doi:10.1016/j.physe.2008.07.019
Wang J, Liu L, Cong SY, Qi JQ, Xu BK (2008) Sens Actuators B 134:1010. doi:10.1016/j.snb.2008.07.010
Ruan QJ, Zhang WD (2008) Mater Lett 62:4303. doi:10.1016/j.matlet.2008.07.012
Polini R, Pamio A, Traversa E (2004) J Eur Ceram Soc 24:1365. doi:10.1016/S0955-2219(03)00592-2
Hien NT, Thuy NP (2002) Phys B 319:168. doi:10.1016/S0921-4526(02)01118-3
Therese GHA, Dinamani M, Kamath PV (2005) J Appl Electrochem 35:459. doi:10.1007/s10800-004-8346-2
Vladimirova E, Vassiliev V, Nossov A (2001) J Mater Sci 36:1481. doi:10.1023/A:1017548813705
Lee S, Lee KS, Woo SK, Kim JW, Ishihara T, Kim DK (2003) Solid State Ionics 158:287. doi:10.1016/S0167-2738(02)00821-4
Manoharan SS, Patil KC (1993) J Solid State Chem 102:267. doi:10.1006/jssc.1993.1031
Woo K, Choi GJ, Sim SJ, Cho YS, Kim YD (2000) J Mater Sci 35:4539. doi:10.1023/A:1004868621334
Lu H, Tong J, Cong Y, Yang W (2005) Catal Today 104:154. doi:10.1016/j.cattod.2005.03.078
Liu J, Co AC, Paulson S, Birss VI (2006) Solid State Ionics 177:377. doi:10.1016/j.ssi.2005.11.005
Leontiou AA, Ladavos AK, Giannakas AE, Bakas TV, Pomonis PJ (2007) J Catal 251:103. doi:10.1016/j.jcat.2007.07.012
Kishimoto H, Sakai N, Horita T, Yamaji K, Brito ME, Yokokawa H (2008) Solid State Ionics 179:1338. doi:10.1016/j.ssi.2007.12.009
Pena MA, Fierro JLG (2001) Chem Rev 101:1981. doi:10.1021/cr980129f
Itagaki Y, Mori M, Hosoya Y, Aono H, Sadaoka Y (2007) Sens Actuators B 122:315. doi:10.1016/j.snb.2006.06.001
Zhao M, Peng H, Hu JF, Han ZX (2008) Sens Actuators B 129:953. doi:10.1016/j.snb.2007.10.012
Wang J, Wu FQ, Shi KH, Wang XH, Sun PP (2004) Sens Actuators B 99:586. doi:10.1016/j.snb.2004.01.012
Songn P, Wang Q, Zhang Z, Yang ZX (2010) Sens Actuators B 147:248. doi:10.1016/j.snb.2010.03.006
Fan HT, Xu XJ, Ma XK, Zhang T (2011) Nanotechnology 22:115502. doi:10.1088/0957-4484/22/11/115502
Liu L, Zhang T, Qi Q, Zhang L, Chen WY, Xu BK (2007) Solid-State Electron 51:1029. doi:10.1016/j.sse.2007.05.016
Song P, Hu JF, Qin HW, Zhang L, An K (2004) Mater Lett 58:2610. doi:10.1016/j.matlet.2004.03.028
Zhang L, Hu J, Song P, Qin H, Liu X, Jiang M (2005) Phys B 370:259. doi:10.1016/j.physb.2005.09.020
Huang SX, Qin HW, Song P, Liu X, Li L, Zhang R, Hu J, Yan H, Jiang M (2007) J Mater Sci 42:9973. doi:10.1007/s10853-007-1991-1
Liu X, Cheng B, Qin HW, Song P, Huang SX, Zhang R, Hu JF, Jiang MH (2008) Sens Actuators B 129:53. doi:10.1016/j.snb.2007.07.102
Pokhrel S, Huo LH, Zhao H, Gao S (2007) Sens Actuators B 122:321. doi:10.1016/j.snb.2006.06.003
Wang J, Zhang P, Qi JQ, Yao PJ (2009) Sens Actuators B 136:399. doi:10.1016/j.snb.2008.12.056
Augustin CO, Selvan RK, Nagaraj R, Berchmans LJ (2005) Mater Chem Phys 89:406. doi:10.1016/j.matchemphys.2004.09.028
Li KY, Wu FQ, Wang DJ, Xie TF, Li TJ (2001) Mater Chem Phys 71:34. doi:10.1016/S0254-0584(00)00524-1
Li SD, Jing LQ, Fu W, Yang LB, Xin BF, Fu HG (2007) Mater Res Bull 42:203. doi:10.1016/j.materresbull.2006.06.010
Tas AC, Majewski PJ, Aldinger F (2000) J Am Ceram Soc 83:2954. doi:10.1111/j.1151-2916.2000.tb01666.x
Karpinski J, Schwer H, Meijer GI, Conder K, Kopnin EM, Rossel C (1997) Physica C 274:99. doi:10.1016/S0921-4534(96)00670-3
Cizauskaite S, Kareiva A (2008) Cent Eur J Chem 6:456. doi:10.2478/s11532-008-0045-0
Gomes J, Pires AM, Serra OA (2006) J Fluoresc 16:411. doi:10.1007/s10895-006-0090-z
Kale GM, Jacob KT (1989) Solid State Ionics 34:247. doi:10.1016/0167-2738(89)90450-5
Ahn MW, Park KS, Heo JH, Park JG, Kim DW, Choi KJ, Lee JH, Hong SH (2008) Appl Phys Lett 93:263103. doi:10.1063/1.3046726
Li JG, Kou XL, Qin Y, He HY (2002) Phys Stat Sol (a) 191:255. doi:10.1002/1521-396X(200205)191:1<255:AID-PSSA255>3.0.CO;2-N
Xu JQ, Pan QY, Shun YA, Tian ZZ (2000) Sens Actuators B 66:277. doi:10.1016/S0925-4005(00)00381-6
Guo PF, Pan HB (2006) Sens Actuators B 114:762. doi:10.1016/j.snb.2005.07.040
Yamazoe N (1991) Sens Actuators B 5:7. doi:10.1016/0925-4005(91)80213-4
Zhao L, Qi JQ, Wang J, Yao PJ (2012) Meas Sci Technol 23:085101. doi:10.1088/0957-0233/23/8/085101
Liu X, Cheng B, Hu JF, Qin HW, Jiang MH (2008) Sens Actuators B 133:340. doi:10.1016/j.snb.2008.02.033
Chen YC, Chang YH, Chen GJ, Chai YL, Ray DT (2003) Sens Actuators B 96:82. doi:10.1016/S0925-4005(03)00489-1
Chen T, Zhou ZL, Wang YD (2009) Sens Actuators B 143:124. doi:10.1016/j.snb.2009.09.031
Acknowledgements
This subject was supported by the National Natural Science Foundation of China (61176068, 61131004). The authors thank Mr. Xing-Ru Chen for his help in the experiments. The authors are grateful to the anonymous reviewers for their valuable corrections and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, PJ., Wang, J., Chu, WL. et al. Preparation and characterization of La1−x Sr x FeO3 materials and their formaldehyde gas-sensing properties. J Mater Sci 48, 441–450 (2013). https://doi.org/10.1007/s10853-012-6758-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6758-7