Abstract
In the present article, a thermodynamic description of the ternary Al–Fe–Ta system is proposed, being obtained by CALPHAD modelling based on assessed experimental information from literature and our own experimental results. Dedicated experiments were performed to complement literature data on phase equilibria and thermodynamic properties. As-cast and annealed samples were prepared and analysed by means of SEM/EDS, XRD, DTA and DSC techniques, as well as isoperibolic dissolution calorimetry. The experiments revealed that three binary phases have large ternary extensions: the C14 Laves phase based on TaFe2 dissolves up to about 56 at.% Al, the μ or D85 phase dissolves up to about 39 at.% Al and the σ phase based on Ta2Al dissolves up to 12 at.% Fe. The ternary ordered Heusler phase L21 (the MnCu2Al crystal structure type) is modelled as a stable phase such that it forms through a second-order ordering process at 1122 K from the ordered B2 phase of composition Ta0.04Fe0.50Al0.46 involving the substitution of Al with Ta atoms and reaches TaFe2Al stoichiometry at ambient temperatures. The elaborated thermodynamic description was applied to calculate selected phase equilibria as to provide a comparison between calculated and experimental results. The calculations are shown to reproduce reasonably the experimental data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Al–Fe–Ta system is of interest for iron aluminides reinforced with the Laves phase λ-(TaFe2) and/or Heusler phase (L21) [1–5], as well as for eutectic (Fe) + λ [6] and various magnetic alloys. Ordered Al–Fe–Ta alloys strengthened by λ show good creep behaviour, high strength and good corrosion resistance at elevated temperatures [2, 4, 7–9]. They can be prepared by ingot metallurgy and are attractive materials since the mechanical properties can be optimised through adjustment of the Ta content, which controls the volume fraction of the Laves phase, and adjustment of the Al content, which controls the atomic order in the Fe–Al matrix [7]. A consistent thermodynamic description of the ternary system is required for microstructure optimization of such materials [5]. The description proposed 20 years ago [10] is inconsistent with latest research results.
The study reported here aimed to construct the phase diagram for a wide temperature range and to provide an improved thermodynamic description of the entire Al–Fe–Ta system. Published data on phase equilibria and thermodynamic properties for this system were analysed and complemented by our own experimental results. The assessed literature data, experimental results obtained in the present work and recently published descriptions of the constituent binary systems Al–Fe [11], Al–Ta [12] and Fe–Ta [13] were used for CALPHAD modelling (Computer Coupling of Phase Diagrams and Thermochemistry).
The article is structured as follows: in the second and third section, the experimental data are presented with main emphasis on the measurements performed within the frame of this study. The forth section contains a brief description of the thermodynamic models used for expressing the Gibbs free energy of the individual phases of the alloy system and a description of the optimization procedure. The resulting thermodynamic database is given in Appendix. In the fifth section, a large number of calculations performed with the elaborated thermodynamic description, including the complete Scheil’s reaction scheme, liquidus and solidus projections, isothermal sections and selected isopleths. The calculations are discussed in comparison with experimental data regarding phase equilibria of major interest.
Literature data for the ternary Al–Fe–Ta system
The Al–Fe–Ta system was recently assessed by Ghosh [14], who substantially enhanced previous reviews [15–17]. Table 1 summarizes the crystallographic data for all known phases of the system, taken from [11–14]. As shown in [14], the main composition range studied so far was limited to the Al- and Ta-lean region with less than 35 at.% Al and 50 at.% Ta, respectively. Besides this region, experimental investigations were reported in [18, 19] for the rapidly solidified Ta10Fe20Al70 alloy that formed the metastable icosahedral i-phase with the quasilattice constant of a = 460.0 ± 0.3 pm. Heating at 10 K min−1 gave rise to decomposition of the i-phase into Fe4Al13 and TaAl3 (ε) at about 900 K [18].
The partial isothermal section at T = 1273 K was investigated experimentally by Hunt and Raman [20], Risanti and Sauthoff [7] and further modified by Ghosh [14]. At this temperature, the phases λ and μ present in the binary constituent system Fe–Ta display extensive solubility for Al replacing Fe. Interestingly, above references indicate a termination of the two single-phase fields λ and μ at a characteristic Al content, both phases re-appearing as λ′ and μ′, once the Al content increases further. However, in recent publications [23, 24], Risanti and Sauthoff attributed this to impurity effects and proposed that the single Laves phase field extends to 50 at.% Al without interruption.
Other data on two-phase equilibria between (Fe) and λ were reported by Reviere et al. [6] and Von Keitz et al. [21] obtained from the as-cast samples, Risanti and Sauthoff at 1473 and 1073 K [3, 7, 22–24], as well as Palm et al. [9] at 1073 and 873 K. For single Laves phase samples, the reported differential thermal analyses (DTA) showed that the addition of Al led to a slight reduction of melting temperature and X-ray diffraction (XRD) measurements revealed an increase of the lattice parameters [21].
Anthony and Fultz [25] experimentally investigated the effect of Ta on the critical temperature of the D03–B2 transformation in four alloys (Fe74Al26, Ta1Fe73Al26, Ta2Fe72Al26 and Ta4Fe70Al26) by XRD and DTA. They reported an increase of the transformation temperature from 823 K for the binary sample to 858 K with addition of 1 at.% Ta, above which it remains at the same level due to the limited solubility of Ta in D03. Consequently, the authors [25] pointed out the solubility limit of Ta in D03 to be less 1 at.%. Unlike in [7, 22], the latest publications of Risanti and Sauthoff [23, 24] confirmed the above trend: the transformation temperature reaches 873 K for an alloy Ta2Fe73Al25 and decreases to 853 K with further increasing the Ta content to 6 at.% (the Al content is constant). The latest papers [2, 9, 24] indicated that at 923 K fine coherent L21 particles precipitated in the alloy Ta2Fe73Al25 under creep load applied for 600 h. In this temperature domain, phase equilibria for alloys along the section Fe3Al–Ta (0 to 6 at.% Ta) are quite complex and require further investigation. Already in the constituent binary Al–Fe system [11] (see also Fig. 11a of the present study) order–disorder transformations in the vicinity of the Fe3Al composition are sensitively depending on the Al content: in cooling the alloy Fe74Al26, the sequence of ordering transformations is B2\D03 (second-order at T = 853 K) followed by D03\D03 + A2fm Footnote 1 (first-order at T = 818 K), while alloy Fe75Al25 gives another transformation sequence, i.e. B2\A2fm + B2 (first-order at T = 854 K) and A2fm + B2\A2fm + D03 (a second-order invariant reaction B2 ⇆ A2fm + DO3 at T = 839 K).
So far, no experimental data regarding thermodynamic properties of ternary Al–Fe–Ta alloys were reported in literature. Bozzolo et al. [26] calculated the formation energy of B2 ternary alloys Ta x Fe50−x Al50 and Ta x Fe50Al50−x (x = 0,…, 1.39) by the Bozzolo–Ferrante–Smith muffin-tin orbital method. For the latter alloys, the partial ordering energy was also calculated by Mekhrabov and Akdeniz [27] applying the quasi-chemical method combined with electronic theory in the pseudo-potential approximation.
Additional experiments
Preparation of samples
Aiming to examine phase equilibria in the Al–Fe–Ta system, in total, 19 alloys were prepared by arc melting with a non-consumable tungsten electrode on a water-cooled copper hearth under purified argon. The samples, with a mass of 15 g each, solidified at a cooling rate of about 100 K s−1. The initial materials were bulk Al (purity 99.999 wt%, Kirov’s Plant, Volkhov, Russia), bulk Ta (purity 99.97 wt%, Ulbinskiy Metallurgical Plant, Russia) and carbonyl iron (purity 99.95 wt%, P/B 4019, Russia), as well as master-alloys (at.%) Ta43.4Al56.6, Ta25.2Al74.8 and Ta51.3Fe48.7. The master alloys were prepared using above-mentioned pure elements and the same preparation procedure.
The sample compositions are listed in Table 2 and pertain to four groups: (i) samples located in vicinity of 13 at.% Ta (samples no. 1–10); (ii) samples consisting of mainly Laves phase and containing about 32 at.% Ta (samples no. 11–14); (iii) samples consisting of mainly μ phase and containing about 52 at.% Ta (samples no. 16–18); and (iv) samples with about 13 at.% Fe (samples no. 5, 11, 15, 16 and 19). An alloy with composition Ta2.0Fe73.5Al24.3C0.2 (sample no. 20) received from Max-Planck-Institut für Eisenforschung GmbH (Düsseldorf, Germany) for casting trials was also investigated. Moreover, this material was unidirectionally solidified in a Bridgman furnace [28] and quenched after planar growth and dendritic growth. This allowed to evaluate equilibrium and non-equilibrium compositions of the bcc A2(B2) and liquid phases.
As measured by reducing extraction in a Ni bath followed by chromatography, the oxygen content in the samples ranged from 0.03 to 0.07 wt% and the contamination by N and H was lower than the detection limit (about 0.001 wt% N and 0.003 wt% H). Carbon contamination was determined to range from 0.02 to 0.04 wt%, originating mainly from the Fe and Ta used. The samples were chemically analysed and studied in the as-cast state as well as after annealing at selected temperatures (see Table 2) by means of scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDS), XRD, DTA, differential scanning calorimetry (DSC) and isoperibolic dissolution calorimetry.
Annealing treatments of all samples were performed at a defined temperature close to the alloy’s solidus (40–70 K below Tsol) for 5–8 h in a resistance furnace with a tungsten heater in argon atmosphere gettered by Ti, Zr or Hf cuttings. This annealing treatment is called ‘subsolidus annealing’ further on. The cooling rate after annealing ranged around 3 K s−1. Several selected samples were further annealed at 1123 K for 744 h, at 923 K for 482 h and at 823 K for 362 h in a tube furnace. The samples were sealed under Ar in quartz ampoules and quenched in water after completed annealing.
SEM/EDS analysis of samples
SEM/EDS were carried out in a ZEISS-LEO-type Gemini 1550 microscope (Karl Zeiss, Inc., Germany) equipped with an energy dispersive X-ray analysis system (INCA Microanalysis system, Oxford Instruments). Phase composition measurements were carried out at an accelerating voltage of 15 kV and a working distance of 10.5 mm, mainly by means of spot measurements. The integral composition was determined as average from at least 3 EDS measurements taken within rectangular frames of adequate size, e.g. 600 μm × 400 μm. The acquired EDS spectra were evaluated against an own standard with composition close to the equiatomic TaFeAl, initially determined by wet chemical analysis. The averaged chemical composition of the alloys and the coexisting phases in all as-cast and annealed samples are listed in Table 2. The table also includes the composition of eutectic structure constituents being measured by EDS within frames of adequate size, whenever applicable.
SEM micrographs of the as-cast samples (left column) and samples annealed at subsolidus temperatures (right column), all from group (i) containing about 13 at.% Ta, reveal the C14 Laves (λ) phase as major constituent (Fig. 1).Footnote 2 In the as-cast condition, all samples show primary dendrites of the Laves phase, i.e. up to 73.4 at.% Al. The two-phase A2(Fe)(black) + λ(white) eutectic is clearly observed in the sample no. 3 (Fig. 1a, left). Sample no. 7 displays primary Laves dendrites and interdendritic Fe2Al5 grains decorated with a fine, terminal eutectic (Fig. 1c, left). After subsolidus annealing, the samples no. 3 and no. 1 shown in Fig. 1a, b on the right side display a two-phase microstructure consisting of A2(Fe)(black) + λ(white), the annealed sample no. 7 (Fig. 1c, right) consists of Fe2Al5(black) + λ(white), while sample no. 5 (Fig. 1d, right) is composed of three phases Fe4Al13(black) + λ(grey) + ε(white).
SEM/BSE micrographs of the as-cast alloys (left column) and samples annealed at subsolidus temperatures (right column) reveal the following microstructure constituents: A2(Fe)(dark) + λ(white) in sample no. 3 (a) and no. 1 (b); Fe2Al5(dark) + λ(white) and tiny eutectic grains in sample no. 7 (c) and Fe4Al13(dark) + λ(grey) + ε(white) in sample no. 5 (d). Few very bright particles in (a–c) are carbide inclusions originated from carbon impurities. Annealing conditions and phase compositions are given in Table 2
The microstructures of the as-cast and annealed samples (subsolidus annealing) from group (ii) containing about 32 at.% Ta are displayed in Fig. 2 and indicate that the homogeneity range of the C14 Laves phase is particularly extended. Few bright particles were identified to be fcc (Ta1−x Fe x )C1−y carbide inclusions [29], originating from carbon impurities. Sample no. 11 (Fig. 2d) contains about 15 vol.% of ε, 85 vol.% of λ and traces of Fe4Al13. The last phase is found both in the as-cast condition and also after annealing for 6 h at 1723 K. Since the sample composition is practically in the ε–λ tie-line, we can assume that Fe4Al13 is formed as non-equilibrium phase upon cooling below 1420 K. The microstructures of samples from group (iii) with about 53 at.% Ta are displayed in Fig. 3 and indicate the wide homogeneity range of the μ phase. Sample no. 18 is nearly composed of single μ phase in the as-cast state and certainly after subsolidus annealing (Fig. 3a). Sample no. 17 with 23.6 at.% Al consists of μ + σ, the primary and major phase is the plate-like μ, the plates being separated by very thin layers of σ (Fig. 3b). The as-cast sample no. 16 contains primary σ dendrites enveloped by μ. After subsolidus annealing, its microstructure contains coarsened σ that appears more rounded being embedded in the μ phase matrix (Fig. 3c).
SEM/BSE micrographs of the as-cast alloys (left column) and samples annealed at subsolidus temperatures (right column) reveal the following microstructure: λ(light grey) with significant microsegregation in samples no. 14 (a), no. 13 (b) and no. 12 (c); λ(light grey) + ε(dark grey) in sample no. 11 (d). Black areas in (b) and (c) correspond to porosity. Very bright particles are carbide inclusions originated from carbon impurities. In (a) and (b), small fraction of non-equilibrium μ phase and in (d) traces of non-equilibrium Fe4Al13 phase formed below 1420 K during cooling were detected. Annealing conditions and phase compositions are given in Table 2
SEM/BSE micrographs of the as-cast alloys (left column) and samples annealed at subsolidus temperatures (right column) reveal the following microstructure constituents: μ(grey) in sample no. 18 (a); μ(grey) + σ(bright) in samples no. 17 (b) and no. 16 (c). In (b) and (c), small fraction of non-equilibrium λ precipitates formed during cooling was detected by XRD. Annealing conditions and phase compositions are given in Table 2
Sample no. 20 (Ta2.0Fe73.5Al24.3C0.2) shows the expected dendritic A2(Fe) solidification structure with pronounced segregation of Ta (Fig. 4a). The same alloy was subjected to unidirectional solidification (UDS) followed by quenching: the microsegregation pattern is clearly visible in the transverse section through the quenched mushy zone in Fig. 4b. In the interdendritic region, there is a minor amount of carbide particles (too few for detection by XRD) and substantial amount of non-equilibrium Laves phase formed due to the strong segregation of Ta. The carbides seem to be fcc (Ta1−x Fe x )C1−y , where x ≈ 0.05 and y ≈ 0.3, as reported by Schneider et al. [29] for the alloy Ta2.0Fe71.0Al26.0C1.0. After annealing at 1673 K for 5 h, the non-equilibrium Laves phase dissipated and the microstructure consists of few large A2(Fe) grains and a small amount of tiny (1,…, 5 μm) carbide particles (Fig. 4c). After further annealing at 1123 K for 362 h and 923 K for 482 h, the sample shows plate-like precipitates inside the (Fe) matrix, identified by XRD as Laves phase. After annealing at 823 K for 362 h (subsequent to annealing at 1673 K for 5 h followed by quenching in Ga), no precipitates are visible in SEM (Fig. 4f), but a little amount (2.3 %) of the Heusler phase L21 was identified by XRD (see ‘XRD analysis of samples’ section).
SEM/BSE micrographs of sample no. 20 (Ta2.0Fe73.5Al24.3C0.2) in the as-cast state (a) and after unidirectional solidification (UDS) in transverse section (b); after annealing UDS sample at 1673 K for 5 h (c), at 1123 K for 362 h (d), at 923 K for 482 h (e) and at 823 K for 362 h (f). Very bright particles/areas correspond: a to carbides and non-equilibrium Laves phase, b to quenched liquid enriched with Ta, c and f to carbides, d and e to Laves phase precipitates. In f, no precipitates were visible in SEM, but XRD (see ‘XRD analysis of samples’ section) reveals the presence of A2 and L21
The microstructure of samples no. 1–3 was investigated after annealing at 1123 K for 744 h and at 923 K for 482 h (Fig. 5a–c). The microstructure consists of mainly a matrix of A2 or B2 and grains of Laves phase. The X-ray diffractogram of sample no. 1 shows that only traces of the Heusler phase L21 may be assumed to form during annealing at 923 K for 482 h (see ‘XRD analysis of samples’ section).
SEM/BSE micrographs of samples annealed at 1123 K for 744 h (left column) and at 923 K for 482 h (right column) reveal the following microstructure constituents: λ(bright) and (Fe)-matrix (black). In (c)XRD (see ‘XRD analysis of samples’ section) revealed the presence of L21, assumed to be nanosized precipitates inside the B2(Fe) matrix
XRD analysis of samples
XRD measurements were carried out in a DRON-3 (Burevestnik, Inc., St. Petersburg, Russia) diffractometer on powder samples or on coarse grained samples for Fe-rich alloys. The X-ray diffractograms of the as-cast samples and samples annealed at subsolidus temperatures (see Table 2) were recorded using the Cu–Kα filtered radiation at an angular step of 2θ = 0.05° and an exposure of 8 s. For the samples annealed at 1123 K for 744 h, at 923 K for 482 h and at 823 K for 362 h, the Cu–Kα1 monochromatic radiation was used, and the exposure time was increased up to 30 s. The obtained data are also summarized in Table 2. The phase constituents identified by XRD are fully consistent with the results from SEM/EDS analysis.
X-ray diffractograms were carefully analysed with respect to the presence of the Heusler phase in samples no. 1 and 20 after low temperature annealing treatments. Unfortunately, little is known about the lattice parameters and site occupation of the L21 phase TaFe2Al; based on theoretical calculations, Mekhrabov and Akdeniz [27] predicted that Ta atoms preferentially substitute the Fe sublattice sites in Ta x Fe50Al50−x , while Bozzolo et al. [26] suggested the substitution of Al sublattice sites by Ta for Ta x Fe50−x Al50 and Ta x Fe50Al50−x alloys. Anthony and Fultz [25] experimentally established that in the L21 phase, Ta atoms occupy the 4(b) Wyckoff sites (1/2, 1/2, 1/2), rather than 8(c) with Fe or 4(a) with Al. It is worth noting that only the difference in the occupation of 4(a) and 4(b) Wyckoff positions distinguishes the L21 and the B2 structures. Hence, the smaller the difference of the averaged atomic weights in 4(a) and 4(b) sites, the smaller the intensity of the superstructure reflections with odd indexes (111), (311), etc., characteristic for L21, will be. In the present study, the lattice atom occupation for the L21 phase was taken from [25] with Al, Fe, Ta on the 4(b) position. The site fractions for this phase for the temperatures of interest were obtained by thermodynamic calculations by means of the proposed description.
By means of illustration, Fig. 6 shows fragments of the full profile analysis by Powder Cell (version 2.4) software for sample no. 1 (Ta13.4Fe51.2Al35.4) annealed at 923 K for 482 h and sample no. 20 (Ta2.0Fe73.5Al24.3C0.2) annealed at 923 K for 482 h and at 823 K for 362 h. In Fig. 6a, the characteristic peaks (111) and (311) of L21 do not appear unambiguously from the background and accordingly the fraction of the L21 phase is either small or the phase is entirely absent. The Rietveld refinement for the sample no. 20 annealed at 923 K for 482 h (Fig. 6b) shows no L21 phase, but only 95 % B2 + 5 % λ, while the same sample annealed at 823 K for 362 h (Fig. 6c) is composed of ~98 % A2 + ~2 % L21 with Laves phase being absent.
Lattice parameters of the λ and μ phases were evaluated as function of the aluminium content. The results are shown in Fig. 7: the solubility of Al in λ extends up to 52 at.% Al and up to 36 at.% in μ, both at subsolidus temperatures. The lattice parameters of the λ phase are in excellent agreement with data reported by Von Keitz et al. [21] for the composition range from 0 to 35 at.% Al.
(Colour online). Variation of lattice parameters as a function of the Al content in sample series consisted mainly of λ phase (a) and μ phase (b). In (a) data reported by Von Keitz et al. [21] are also included
DTA and DSC analysis of transformation temperatures
Temperatures of solid-state and solid–liquid phase transformations for the as-cast samples and samples annealed at subsolidus temperatures were determined by DTA using Sc2O3 crucibles for samples in the range rich in Ta (≥32 at.%) and lean in Al (<40 at.%) and Al2O3 crucibles for all other samples. The high temperature DTA apparatus (max. temperature 2500 K) was equipped with W/W-20Re string thermocouples designed by Kocherzhinskiy et al. [30, 31]. The DTA measurements were performed under high purity He atmosphere with heating and cooling rates of 20 K min−1. The thermocouple was calibrated using the International Temperature Scale of 1990 reference points of Al, Au, Pd, Pt, Rh and additional reference points of Fe and Al2O3. After calibration, the reproducibility of phase transformation temperatures of these references was evaluated to be about ±0.5 %. During the evaluation of phase transformation temperatures from the recorded DTA curves, the practical guide on differential thermal analysis of metals and alloys recommended by the National Institute of Standards and Technology [32] was followed.
DTA measurements were performed first for the as-cast samples. This allowed selecting an appropriate temperature for subsolidus annealing. DTA measurements were repeated for all annealed samples, the results being summarized in Table 3, along with the values calculated using the proposed thermodynamic description of the Al–Fe–Ta system. Hence, we have used the so-called ‘hybrid approach’ that has been applied quite successfully for the study of phase equilibria and phase transformations in many systems. It consists of performing experimental investigations of phase equilibria in parallel with thermodynamic modelling. Therefore, our interpretation of the DTA results in Table 3 was guided by thermodynamic calculations performed throughout the CALPHAD assessment. To underline this, we explicitly included in Table 3 the column ‘Phase transformation’ into the block labelled ‘Results of calculation’.
By means of the illustration, DTA curves recorded upon heating and cooling are displayed in Fig. 8 for few selected samples. The curve of alloy no. 9 is meant to illustrate the resolution DTA signals at heating and cooling rates of 20 K min−1. The complex cascade of transformations, taking place in the temperature range 1369–1445 K within an interval of 76 K, can be well resolved to yield four independent peaks/signals. The resolution is especially good upon cooling. Only the heating curves were used for determining transformation temperatures since undercooling effects were observed during solidification, which were in many cases associated with strong recalescence (see, for example, the DTA curve upon cooling for the alloy no. 18 in Fig. 8).
From the recorded DTA data, it is possible to conclude that the ternary system comprises a quasi-binary eutectic reaction, L ⇆ A2 + λ, at a temperature 1733 K or somewhat higher (see the alloy no. 4 in Table 3). This can be inferred from the fact that the solidus temperatures first rise with increasing Al content compared to the Fe–Ta binary alloys (see [13]), then reach the maximum value in the range of 7–15 at.% Al before decreasing again as the Al content continues to increase. Just the same behaviour is observed for the alloys with 53–54 at.% Ta being close to the homogeneity range of μ-phase (see the alloy no. 18 in Table 3).
A series of DSC measurements was performed with alloys no. 1–3 annealed at 1123 K and alloy no. 20 annealed at 1123, 923 and 823 K using a PyrisTM DSC-7 (PerkinElmer, Inc., Massachusetts, USA) calorimeter operating in temperature range from 300 to 1000 K. The calorimeter was calibrated by measuring the melting point of high purity Al and the heat capacity of a reference sample (sapphire disc) supplied by Perkin-Elmer. For each DSC-run, a sample of 130 ± 10 mg was used. After introducing the sample into the DSC furnace and sealing the furnace tightly, the excess pressure of the inner gas (Ar with purity 6 N) in the furnace automatically rose to about 100 Pa. The heating and cooling rate of 10 K min−1 was found best for observing solid-state transformations. Several heating/cooling cycles were applied for each selected sample, aiming to detect the sequence of ordering reactions. The transformation temperatures determined by DSC are given in Table 3, but the values are rather tentative due to the aspect of the apparent heat capacity curves.
As an example, Fig. 9a, b display the DSC curves for alloy no. 20 in two annealing states at 1123 K and 823 K, respectively. These two states differ significantly owing to the apparent heat capacity (Cp) obtained upon heating in the first heating cycle. We think that this may relate to the presence of the L21 phase in the sample annealed at 823 K, but not in the one annealed at 1123 K. The cooling curves, however, look rather similar with respect to values of the apparent heat capacity. This may be attributed to the dissolution of the majority of the L21 precipitates in the course of heating to 1000 K. The peaks as such are interpreted as follows: in Fig. 9a, the peak at 935 K probably corresponds to the magnetic ordering of the A2-phase. Usually, magnetic transformations should only contribute to the entropic term of the Gibbs free energy; however, a small exothermic effect was found upon cooling and could not be fully understood. In Fig. 9b, the peak at 935 K is absent and only two weak and broad endothermal signals around 740 and 875 K are visible. They are attributed to a sequence of second-order transformations as listed in Table 3, based on thermodynamic calculations.
(Colour online). Apparent heat capacity curves measured by DSC in first heating/cooling cycling run with a rate of 10 K min−1 for the sample no. 20 (Ta2.0Fe73.5Al24.3C0.2) annealed at 1123 K for 744 h (a) and at 823 K for 362 h (b) (after annealing the both samples were quenched in water), as well as DTA curve of this sample annealed at 1673 K for 5 h (followed by furnace cooling at a rate of about 3 K s−1) recorded with a rate of 20 K min−1 (c)
Dissolution calorimetry
Within the frame of the present study, the standard enthalpy of formation at 298 K was determined by isoperibolic drop calorimetry (IDC) on samples annealed at subsolidus temperatures (see Table 2). The selected samples contain as a major constituent the λ phase (samples no. 11–14) and the μ phase (samples no. 16–18). In order to find the sample variance and standard deviation, at least five pieces from each selected for the calorimetric investigation material were dissolved. Similar binary Fe–Ta alloys were measured previously [13]. The method is well described [33, 34] and involves the determination of the molar partial enthalpy of dissolution of pure solid Ni, Fe, Ta and Al, as well as that of the ternary Al–Fe–Ta alloys in liquid Ni by IDC. Each dissolution experiment was started by dropping several samples of pure Ni at 298 K into liquid Ni which is at 1773 ± 5 K. Nickel samples were dropped further after each dropping of Al and Ta1−x−y Fe x Al y alloys to determine the composition-dependent calibration factor. The enthalpy change upon heating Ni from room temperature to 1773 K was calculated by means of the Scientific Group Thermodata Europe (SGTE) database PURE4 [35, 36] yielding a value of 66.87 kJ mol−1. The molar partial enthalpies of dissolution (\( {{\Updelta}}_{\text{diss}} \overline{\text{H}}_{\text{i}} \)) of the samples and of pure Al are given in Fig. 10. For the elements Fe and Ta, the molar partial enthalpies of dissolution are determined in the same conditions, being reported in [13]. In the investigated composition ranges, the \( {{\Updelta}}_{\text{diss}} \overline{\text{H}}_{\text{i}} \) varies linearly with solvent composition. Due to this, the molar partial enthalpies of dissolution (\( {{\Updelta}}_{\text{diss}}\overline{\text{H}}_{\text{i}} \)) of cold samples at infinite dilution (\( {\text{x}}_{\text{Ni}} \to 1 \)) and their standard deviations (\( 2\sigma_{\text{i}} \)) were obtained by means of linear fit of the measured values. The results are summarized in Table 4.
(Colour online). Enthalpy of dissolution of cold samples (298 K) from pure elements Ni, Al and from selected Al–Fe–Ta alloys in liquid Ni at 1773 ± 5 K as function of molar fraction of the solvent. The selected ternary alloys consisted of the λ phase (a) and the μ phase (b) as single or major constituent
The standard enthalpies of formation (Δ298H0) and their 2σ standard deviations for the Fe1−x−y Ta x Al y alloys, referred to bcc-Fe, bcc-Ta and fcc-Al at 298 K, were determined by means of the values from Table 4 and the following expressions:
The obtained values of the enthalpy of formation for one mole of formula unit of an alloy Ta1−x−y Fe x Al y are also presented in Table 4. From these data, a stabilizing effect of Al is evidenced for both ternary λ and μ phases: the standard enthalpies of their formation decreases with increasing Al content from −6 to about −23 kJ mol−1 and from −7 to about −17 kJ mol−1, respectively. The composition dependencies of Δ298H0 for λ-Ta0.32Fe0.68−x Al x and μ-Ta0.53Fe0.47−x Al x are shown and discussed in ‘Thermodynamics properties’ section based on thermodynamic calculations.
Thermodynamic models and optimization
The designation of individual phases, their crystal structure and the sublattice models for solid phases employed in the present thermodynamic description are summarised in Table 1. The Gibbs energy functions for the stable and metastable structures of the pure elements were adopted from the SGTE database compiled by Dinsdale [35, 36]. We used the thermodynamic models for the individual phases stemming from thermodynamic descriptions of the constituent binary systems Al–Fe [11], Al–Ta [12] and Fe–Ta [13]. The calculated phase diagrams of these constituent binary systems are illustrated in Fig. 11. The gas phase was included to allow extrapolation up to temperatures of 6000 K.
Phase diagrams of the constituent binary systems Al–Fe (a), Al–Ta (b) and Fe–Ta (c) calculated by means of the thermodynamic descriptions proposed in Refs. [11], [12] and [13], respectively. Solid lines denote first-order transformations, dotted lines show second-order chemical and dash-dotted lines show second-order magnetic transformations
The model parameters for liquid and all solid phases of the ternary system Al–Fe–Ta were evaluated by searching for the best fit to available experimental phase equilibria and thermodynamic data by means of the PARROT optimiser of the software Thermo-Calc [37]. During the process of optimization for the liquidus and solidus temperatures determined in the present study (see Table 3), as well as for the analogous DTA data reported in Refs. [23, 24] were assigned a weight of 0.75 and 1.5, respectively, since the liquidus temperatures measured by conventional DTA are commonly less accurate than temperatures of solidus (the latter ones are routinely related with clear incipient melting points; for the former, it is difficult to select the actual thermal event [32]). A weight of 0.5 was fixed to the temperatures of incipient melting of the alloys measured by Pirani–Alterthum method [21]. For the tie-lines and tie-triangles of the present study (see Table 2) and [23, 24], a weight of 1.25 was prescribed. All other data from the present study and literature [7, 23–25] were used with a weight equal 1.
For the description of B2, D03 and L21 ordering (bcc-based phases), as well as for L10 and L12 ordering (fcc-based phases), four-sublattice models based on the compound energy formalism were applied, as proposed in [11]. Ordering to B2, D03 or L21 can be accomplished through first- or second-order transformations, depending on the composition (see Fig. 11a). The model expressions for the molar Gibbs energy as functions of temperature and composition are given in references [11, 37, 38]. When applying the models to ordering reactions in the ternary Al–Fe–Ta system, one should bear in mind that the D03 (Fe3Al) phase is the binary limitrophe form of the ternary Heusler L21 (TaFe2Al) phase [39]. With the addition of small amounts of Ta, the Thermo-Calc software starts to label this phase as L21#1, while the ternary Heusler phase is labelled L21#2, according to distinct composition sets. To improve the readability of the reaction scheme and of relevant figures, we replaced L21#1, which has tiny solubility of Ta, with D03 and L21#2 with L21.
The complete thermodynamic database in Thermo-Calc format [37] is listed in the Appendix.
Results of modelling and discussion
Complete reaction scheme
In the Al–Fe–Ta system, chemical and magnetic ordering reactions are present and were included in the Scheil’s reaction scheme (Fig. 12), following the procedure and designation described in [40]. The ordering reactions may be of second-order nature, but can turn into first-order transformations [11, 39–48] at critical composition and temperatures (points S/F) and vice versa (F/S). In order to distinguish between second-order and first-order reactions, the following notations are used, as proposed in [40]. A superscript ‘fm’ denotes ferromagnetic state of a phase. All first-order univariant reactions are represented by solid lines. All second-order univariant reactions are represented by dotted lines for chemical ordering and dash-dotted lines for magnetic ordering. All univariant second-order reactions are labelled such that the disordered and ordered phases are separated by a slash, e.g. B2/D03 + L21 or A2/A2fm + B2. All first-order invariant reactions are contained in boxes drawn with thick lines. All invariant reactions, which involve ordering, are contained in boxes drawn with thin lines.
Reaction scheme down to room temperature for the Al–Fe–Ta system calculated by means of the present thermodynamic description: solid lines denote first-order transformations, dotted lines show second-order chemical and dash-dotted lines show second-order magnetic transformations. The labelling of both, univariant and invariant reactions, is given above
According to the scheme in Fig. 12, the ternary system Al–Fe–Ta hosts 26 first-order four-phase invariant reactions encompassing 18 reactions of U-type, 6 reactions of E-type, 1 reaction of P-type and 1 reaction of M-type (metatectic). The system also contains 10 so-called quasi-binary three-phase invariant reactions among which 8 are of e-type (e1–e3, e5, e10, e11, e15 and e16) and 2 are of p-type (p4 and p6). The compositions of coexisting phases engaged into invariant equilibria with the liquid phase are listed in Table 5.
All other invariant reactions involve chemical and/or magnetic ordering in distinct configurations, similar to the U- and P-type reactions and also to double degenerated D2-type reactions. The classification and notation of degenerated reactions was initially proposed by Lukas et al. [49]. In order to underline that a reaction involves chemical ordering, a ‘C’ is added as a subscript to the conventional reaction symbol, e.g. EC, UC, PC and DC; for magnetic ordering, a ‘M’ is added; and finally for mixed ordering, ‘C-M’ are added, as discussed and proposed in [40]. The reaction scheme also outlines 8 isothermal events that mark a change of character of a univariant reaction from first- to second-order or vice versa (tricritical lines)Footnote 3 These events are labelled S/F1, S/F2,…through S/F7 and F/S1, respectively. Six of them correspond to chemical ordering and two to magnetic ordering. The most high temperature tricritical line S/F1 occurs at 1583 K and corresponds to the change of A2–B2 ordering in the presence of liquid from the second-order univariant equilibrium L + A2/B2 to the first-order univariant equilibrium L + A2 + B2. The only univariant ordering transformation that retains its second-order nature down to room temperature is the B2–B2fm magnetic ordering in the presence of L2 fm1 .
Owing to the limited experimental data, the ternary ordered Heusler L21 phase was modelled as a stable phase taking into account trends from experimental information for the ternary systems Al–Fe–Ti [50] and Al–Co–Nb [51]. In these systems, the homogeneity range of the L21 phase almost reaches the equiatomic FeAl and CoAl composition. Under these assumptions, modelling reveals that the L21 phase starts to form through a second-order ordering process from the B2 phase of composition Ta0.04Fe0.50Al0.46 according to an invariant reaction B2 ⇆ L21 + λ of eutectoid type at 1122 K (eC1) [40]. With decreasing temperature, the composition of the L21 phase shifts towards the TaFe2Al stoichiometry as a result of substitution of Al sites by Ta atoms. At ambient temperature, the TaFe2Al stoichiometry is reached. The L21 phase turns out to participate in equilibria with the Fe3Al-based composition as reported in [6, 7, 9], including the ordered D03 and D0 fm3 forms. Further investigations are, however, needed to understand the thermodynamic stability of the Heusler and Laves phases, as well as the competitive kinetics of their precipitation: the time–temperature diagram presented in [23, 24] suggests that L21 precipitates are metastable and start to dissolve in favour of Laves phase formation at all temperatures from 923 to 1073 K after sufficient annealing time, e.g. 2000 and 10 h, respectively.
Liquidus and solidus surfaces
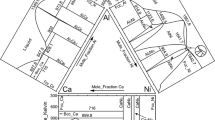
The calculated liquidus surface is displayed in Fig. 13a, b as projection on the Gibbs triangle, along with experimentally observed primary phases and with calculated isotherms, respectively. All invariant four-phase reactions involving the liquid phase are marked as in the reaction scheme (see Fig. 12). The primary λ and μ fields are quite extended towards the Al–Ta side and cover a major part of the liquidus surface. Measured values of the chemical compositions of two-phase eutectics (see data for as-cast samples no. 3, 4, 7–10 and 19 in Table 2) are in proper accordance with the calculated univariant lines (see Fig. 13a). The L ⇆ μ + σ and L ⇆ A2 + λ univariant reaction lines display temperature maxima at points e1 and e5, corresponding to the quasi-binary eutectics. This agrees well with the results of DTA, described in ‘DTA and DSC analysis of transformation temperatures’ section. The position of the quasi-binary reaction e5 is similar to the one reported for the Al–Fe–Nb system [52]. The calculations revealed more complex variation of the liquidus univariant L + λ + μ line in the composition range (5–27)Al–(50-30)Fe–(42–44)Ta (at.%): it changes its character from peritectic L + λ ⇆ μ to eutectic L ⇆ μ + λ with increasing Al content and then passes through two points corresponding to invariant quasibinary reactions (e3 and e2, respectively) with temperature difference of 21 K (see Table 5). Such behaviour of the univariant L – λ – μ groove is related to the presence of two congruent equilibria between liquid and λ at T = 2123 K (29Al–40.5Fe–30.5Ta) and liquid and μ at T = 2095 K (22Al–26Fe–52Ta) marked as ‘C1’ and ‘C2’ in Fig. 13b, respectively.
The calculated solidus surface is shown in Fig. 14a as projection on the Gibbs triangle along with experimental data on single-phase domains and tie-lines. The calculated solidus surface in Fig. 14b includes calculated isotherms. The solidus surface contains 12 tie-triangles corresponding to the four-phase invariant reactions with the liquid phase, as described above. In total, 7 folds are marked by thick solid lines, corresponding to the quasi-binary invariant reactions with the liquid phase. The solidus surface also shows the wide homogeneity range for the phases λ, μ and σ that extend to 56 at.% Al, 39 at.% Al and 12 at.% Fe, respectively. This is in proper agreement with the XRD and EDS measurements described in ‘SM/EDS analysis of samples’ and ‘XRD analysis of samples’ sections.
Isothermal sections
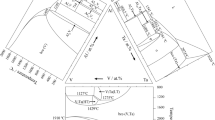
Figures 15, 16 and 17 display a series of calculated isothermal sections: experimentally measured tie-lines and tie-triangles from literature [23, 24] and from the present study are included. For T = 1973 K (Fig. 15a) and T = 1723 K (Fig. 15b), tie-lines data stem from the present study only. For T = 1723 K, the tie-line between A2 and liquid was determined at a planar interface quenched in after unidirectional solidification of the alloy no. 20. The isothermal sections for T = 1423 K (Fig. 15c), T = 1273 K (Fig. 15d) and T = 1073 K (Fig. 15f) contain the tie-lines reported in [23, 24] for samples annealed for 100, 200 and 1000 h, respectively. The isothermal section for T = 1123 K (Fig. 15e) contains the tie-lines experimentally measured in samples no. 1, 2, 3, 8 and 15 annealed for 744 h followed by quenching in water. The directions of all experimental and calculated tie-lines are markedly different, while the phase boundaries are reasonably matched. Our attempts to better reproduce the experimental tie-line directions always led to unacceptable distortions of the phase boundaries, especially for A2 and B2, and were therefore abandoned. It is likely that the experimental tie-lines do not correspond to phase equilibrium, meaning that annealed samples still contain residual micro-segregation inherited from solidification and longer annealing may be required to equilibrate them.
(Colour online). Isothermal sections for the Al–Fe–Ta system at T = 1973 K (a), T = 1723 K (b), T = 1423 K (c), T = 1273 K (d), T = 1123 K (e) and T = 1073 K (f). Lines are calculated phase equilibria by means of the proposed thermodynamic description and tie-lines and tie-triangles assigned by symbols are experimental data from the present study and from [23, 24]. Dotted lines separate ordered from disordered phases (chemical ordering)
(Colour online). Calculated isothermal sections for the Al–Fe–Ta system at moderate and ambient temperatures T = 873 K (a), T = 573 K (b), T = 500 K (c) and T = 298 K (d). Dotted lines separate ordered from disordered phases (chemical ordering) and dash-dotted lines separate the paramagnetic from the ferromagnetic state of A2, B2, D03 and L21
(Colour online). Calculated isopleths for the Al–Fe–Ta system (lines) compared to experimentally determined phase transformation temperatures reported in [13, 21, 23, 24] and in ‘DTA and DSC analysis of transformation temperatures’ section of the present study for 13 at.% Fe (a), Ta15Fe85–Ta12Al88 (b), 32 at.% Ta (c), 53 at.% Ta (d), 15 at.% Al (e) and 35 at.% Al (f). Dotted lines show chemical ordering and dash-dotted lines show magnetic ordering accomplished by second-order transformations
Calculated isothermal sections for T = 873, 573, 500 and 298 K are displayed in Fig. 16a–d, showing the occurrence of ordered D03 and Heusler L21 phases, as well as three-phases fields A2fm + D0 fm3 + L2 fm1 and B2fm + D0 fm3 + L2 fm1 that arise from first-order chemical and magnetic transformations. The univariant phase equilibria with ordered and disordered phases are also outlined by dotted lines for chemical ordering and dash-dotted lines for magnetic ordering. Experimental data for these low temperatures are scarce, comprising only DTA and DSC measurements of the present study and from [7, 23–25], and further experimental studies are required. Figure 16c shows that the Heusler L21 phase approaches the TaFe2Al stoichiometry and both the λ and μ phase fields are present as two islands. Such feature was reported in [7, 14, 20], but for a temperature value of 1273 K. Finally, Fig. 16d shows the calculated isothermal section at room temperature: the ferromagnetic Heusler phase L2 fm1 appears as a ternary line compound with the composition quite close to the TaFe2Al stoichiometry, being the dominant phase in the entire system. Only one three-phase field B2/B2fm + L2 fm1 associated with second-order magnetic transformation remains present at this temperature (see also reaction scheme in Fig. 12), which is shown by dash-dotted line.
Isopleths
Selected isopleths are presented in Figs. 17 and 18, aiming to compare calculated phase transformation temperatures with experimental data from literature, from DTA and DSC measurements reported in ‘DTA and DSC analysis of transformation temperatures’ section. The experimental data points are not always precisely in the plane of the calculated isopleths, but may deviate by maximum 2 at.%. Figure 17b also illustrates the reproducibility of DTA signals for several alloys in the annealed condition obtained in two distinct runs performed at heating and cooling rates of 20 K min−1. The calculated phase transformation temperatures agree with experimentally measured values of the present study, except for the liquidus temperatures of the alloys no. 6, 7, 8 (Fig. 17b) and no. 11 (Fig. 17c). These Al-rich alloys are believed to suffer partly from considerable loss of Al by evaporation when heating the liquid above 1800 K. A further contribution to the observed differences between measured and calculated liquidus temperatures of the Al-rich samples no. 6–8 may relate to a slower kinetics of dissolution (retarded dissolution) of the Al-rich ternary Laves phase, as may be suspected from its high negative enthalpy of formation (Fig. 19a) and refractoriness (see Fig. 14b).
(Colour online). Calculated isopleths for the Al–Fe–Ta system at 25 at.% Al (a), 2 at.% Ta (b) and 7 at.% Ta (c) along with experimentally determined phase transformation temperatures reported ‘DTA and DSC analysis of transformation temperatures’ section and published in [7, 23–25]. Dotted lines show chemical ordering and dash-dotted lines show magnetic ordering accomplished by second-order transformations
(Colour online). Standard enthalpies of formation of Al–Fe–Ta alloys having the λ (a) or the μ (b) phase as single or major microstructure constituent. Symbols are experimental data reported in ‘Dissolution calorimetry’ section, while lines are calculated values by the present thermodynamic description for Ta0.32Fe0.68−x Al x and Ta0.53Fe0.47−x Al x isoconcentration sections. The reference state is fcc-Al, bcc-Fe and bcc-Ta at 298.15 K
The melting temperatures of λ reported by Von Keitz et al. [21] (Fig. 17c), as well as the recent DTA results of Risanti and Sauthoff [7, 23, 24] (Fig. 18), are also reasonably reproduced. In numerous alloys, the temperature associated to A2–B2, B2–L21 and A2–A2fm second-order transformations was clearly detected on the DTA curves, and these data agree well with the calculations (see dotted and dash-dotted lines in Figs. 17a, e, f, 18a, b).
The alloy no. 20 (Ta2.0Fe73.5Al24.3C0.2) as attractive for industry received much attention, aiming to understand precipitation and ordering at potential service temperatures below 900 K. The isopleths displayed in Fig. 18a, b show that below 839 K, this alloy should be composed of A2fm + D03 + L21 and above 860 K of B2 and λ-phase. The disordering transformations upon heating should occur through a second-order invariant reaction A2fm + D03 → B2 + L21 at 839 K (UC2) followed by a first-order invariant reaction A2fm + L21 → B2 + λ at 840 K (U14) and should finish after passing through A2fm + B2 + λ\B2 + λ phase boundary at 860 K. We attempted to verify these transformations by DSC and XRD techniques; however, the complex reaction sequence requires more detailed analysis in future. At this stage, we can only emphasise that the precipitates formed upon annealing at 923 and 1123 K are Laves phase; whereas, after the annealing at 823 K for 362 h, only the nanosized Heusler phase precipitates in A2(Fe) matrix were identified (see Figs. 4, 6), which are in accordance with the thermodynamic calculations.
Thermodynamic properties
Figure 19 displays experimentally measured values of the standard enthalpy of formation of ternary Al–Fe–Ta alloys containing the λ and μ phases as a major microstructure constituent. The calculated standard enthalpy of formation is equally shown as a function of the aluminium content, i.e. for the Ta0.32Fe(0.68−x)Al x and Ta0.53Fe(0.47−x)Al x isoconcentration sections. Both measured and calculated data refer to fcc-Al, bcc-Fe, bcc-Ta at 298.15 K and to identical formula units. Because samples of both series, excluding alloy no. 18 (see Table 2), were not single-phased materials, calculations were performed that comprise both the major phase and its mixtures with potential minor phase constituents. The calculated and measured values agree well to one another for both series of alloys being inside or close to the homogeneity ranges of λ and μ phases. The results show that λ and μ become more stable after dissolution of about 50 and 33 at.% Al, respectively. This is the main reason for their considerable extension into the ternary Al–Fe–Ta system and also explains the existence of C1 and C2 points of their congruent melting/solidification, as well as e1, e2 and e4 maxima in the L + μ + σ, L + λ + μ and L + A2 + λ univariant liquidus lines, respectively (see Figs. 12, 13b).
Summary and conclusions
Based on the literature data and selected key experiments, a new thermodynamic description of the ternary Al–Fe–Ta system has been proposed. The study encompassed
-
experimental analysis of 20 distinct alloys in both as-cast and annealed states by XRD, SEM/EDS, DTA and DSC techniques for the analysis of microstructure constituents, composition of coexisting phases and phase transition temperatures;
-
determination of the standard enthalpies of formation of λ and μ ternary phases by means of isoperibolic dissolution calorimetry;
-
thermodynamic modelling by the CALPHAD method, taking into account assessed experimental information from literature and our own experimental results;
-
formulation of the complete reaction scheme, comprising first-order reactions, as well as chemical and magnetic ordering transformations of second-order nature;
-
thermodynamic calculations of liquidus and solidus surfaces, selected isothermal and vertical sections, and the standard enthalpy of formation of solid alloys in comparison to experimental data.
The investigations lead to the following conclusions: the ternary alloy system Al–Fe–Ta is the host to several intermetallic phases with extended homogeneity ranges (i) the C14 Laves phase λ based on TaFe2 can dissolve up to 56 at.% Al, (ii) the D85 μ phase can dissolve up to 39 at.% Al, (iii) the σ phase based on Ta2Al can dissolve up to 12 at.% Fe and finally (iv) the ternary Heusler L21 phase presumably forms in the course of a second-order ordering reaction B2 → L21 + λ of eutectoid type at 1122 K in the vicinity of Ta0.04Fe0.50Al0.46 and shifts with decreasing temperature towards the ideal TaFe2Al stoichiometry.
The proposed CALPHAD description can reproduce major part of experimental data on phase equilibria. It also yields reliable values for the standard enthalpy of formation of solid Al–Fe–Ta phases. The complete database attached will serve as a guide for other scientists to operate with Al–Fe–Ta materials and design their future experiments or even improve the description and clarify still open questions related to the formation of the Heusler phase.
Notes
The A2, B2, D03 and L21 phases in para- and ferromagnetic states are treated as different phases due to dissimilar point group symmetry. To distinguish between the different states, the ferromagnetic state is marked with superscript ‘fm’ throughout the article. The back slash ‘\’ denotes crossing of a phase boundary.
References
Sauthoff G (2000) Intermetallics 8:1101
Hanus P, Bartsch E, Palm M, Krein R, Bauer-Partenheimer K, Janschek P (2010) Intermetallics 18:1379
Risanti DD, Sauthoff G (2005) Mat Sci Forum 475–479:865
Hotař A, Palm M (2010) Intermetallics 18:1390
Yoshimi K, Hanada S (1997) JOM 49:46
Reviere R, Sauthoff G, Johnson DR, Oliver BF (1997) Intermetallics 5:161
Risanti DD, Sauthoff G (2005) Intermetallics 13:1313
Palm M (2009) Fe-Al materials for structural applications at high temperatures: current research at MPIE. Int J Mat Res 100:277–287
Palm M, Krein R, Milenkovic S, Sauthoff G, Risanti D, Stallybrass C, Schneider A (2007) Mat Res Soc Symp Proc 980:II01
Kaufman L (1991) Calphad 15:261
Sundman B, Ohnuma I, Dupin N, Kattner UR, Fries SG (2009) Acta Mater 57:2896
Witusiewicz VT, Bondar AA, Hecht U, Zollinger J, Petyukh VM, Fomichov OS, Voblikov VM (2010) Intermetallics 18:92
Witusiewicz VT, Bondar AA, Hecht U, Voblikov VM, Fomichov OS, Petyukh VM, Rex S (2011) Intermetallics 19:1059
Ghosh G (2008) In: Effenberg G, Ilyenko S (eds) Landolt–Börnstein, numerical data and functional relationships in science and technology, (New Series) Group IV: Martinsen W (ed) Physical chemistry, ternary alloy systems. Phase diagrams, crystallographic and thermodynamic data critically evaluated by MSIT, Vol. 11D1. Springer, Berlin, p 213
Kumar KS (1990) Int Mater Rev 35:293
Raghavan V (1992) In: Phase diagrams of ternary iron alloys, Part 6A. Indian Institute of Metals, Calcutta, p 187
Ghosh G (1992) In: Petzov G, Effenberg G (eds), Ternary alloys: a comprehensive compendium of evaluated constitutional data and phase diagrams, Vol. 5. VCH Verlaggeselschaft mbH, Weinheim, p 449
Tsai AP, Inoue A, Masumoto T (1988) Jpn J Appl Phys 27:L5
Srinivas V, McHenry ME, Dunlap RA (1989) Phys Rev 40B:9590
Hunt CR, Raman A (1968) Z Metallkde 59:701
Von Keitz A, Sauthoff G, Neumann P (1998) Z Metallkde 89:803
Risanti DD, Sauthoff G (2006) Entwicklung ferritischer Eisen-Aluminium-Tantal-Legierungen mit verstärkender Laves-Phase mit höchster Zeitstandfestigkeit in korrosiven Atmosphären. DFG Abschlussbericht
Risanti DD (2010) Development of ferritic Fe–Al–Ta alloys with strengthening Laves phase for high temperature applications. Dissertation. RWTH Aachen
Risanti DD, Sauthoff G (2011) Intermetallics 19:1727
Anthony L, Fultz B (1995) Acta Metall Mater 43:3885
Bozzolo GH, Noebe RD, Amador C (2002) Intermetallics 10:149
Mekhrabov AO, Akdeniz MV (1999) Acta Mater 47:2067
Drevermann A, Pickmann C, Tiefers R, Zimmermann G (2004) Ultrasonics 42:105
Schneider A, Falat L, Sauthoff G, Frommeyer G (2003) Intermetallics 11:443
Kocherzhinsky JuA (1971) Proc Third ICTA (Davos) 1:549
Kocherzhinsky JuA, Shishkin YeA, Vasilenko VI (1971) Apparatus for differential thermal analysis with temperature-sensing thermocouple up to 2200 °C. In: Ageev NV, Ivanov OS (eds) Nauka, Moskow, pp 245-249 (in Russian)
Boettinger WJ, Kattner UR, Moon K-W, Perepezko JH (2006) DTA and heat-flux DSC measurements of alloy melting and freezing, NIST recommended practice guide. NIST, Special publication 960-15
Witusiewicz VT, Sommer F, Mittemeijer EJ (2003) Met Mater Trans 34B:209
Witusiewicz VT, Fries SC, Hecht U, Drevermann A, Rex S (2006) Int J Mater Res 97:556
Dinsdale AT (1991) SGTE data for pure elements. Calphad 15:317
PURE4 SGTE database. http://www.thermocalc.se/Products/Databases.html; http://www.thermocalc.com/res/Manuals/tc_database_guide.pdf
Sundman B, Jansson B, Andersson J-O (1985) Calphad 9:153
Lukas HL, Fries SG, Sundman B (2007) Computational thermodynamics. Cambridge Univ. Press, Cambridge
Ohnuma I, Schön CG, Kainuma R, Inden G, Ishida K (1998) Acta Mater 46:2083
Witusiewicz VT, Bondar AA, Hecht U, Velikanova TY (2011) J Phase Equ Diff 32:329
Bean CP, Rodbell DS (1962) Phys Rev 126:104
Stout JW (1948) Phys Rev 74:605
Laughlin DE, Srinivasan K, Tanase M, Wang L (2005) Scripta Mat 53:383
Allen SM, Cahn JW (1982) Bull Alloy Phase Diagr 3:287
Inden G (1982) Bull Alloy Phase Diagr 2:412
Chang YA (1989) Bull Alloy Phase Diagr 10:513
Talanquer V, Varea C, Robledo A (1989) Phys Rev B 39:7030
Matsumura S (1998) Z Metallkde 89:814
Lukas HL, Henig E-T, Petzow G (1986) Z Metallkde 77:360
Palm M, Lacaze J (2006) Intermetallics 14:1291
Dovbenko O, Stein F, Palm M, Prymak O (2010) Intermetallics 18:2191
Prymak O, Stein F (2010) Intermetallics 18:1322
Acknowledgments
The authors thank L. A. Duma, V. V. Garbuz, V. A. Petrova and A. Genau for technical assistance, and would like to express their gratitude for the financial support from the German Federal Ministry of Research (Contract FKZ 50WM0843) and the National Academy of Science of Ukraine (Project III-6-10: 0110U002347).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Thermodynamic database of Al–Fe–Ta system in Thermo-Calc format.






Rights and permissions
About this article
Cite this article
Witusiewicz, V.T., Bondar, A.A., Hecht, U. et al. Experimental study and thermodynamic modelling of the ternary Al–Fe–Ta system. J Mater Sci 48, 377–412 (2013). https://doi.org/10.1007/s10853-012-6755-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6755-x