Abstract
Solid and hollow CoO nanospheres were synthesized by solvothermal method with oleic acid as reactant and SiO2 as template. Each sample of high-purity CoO was characterized by X-ray diffraction, field-emission scanning electron microscopy, transmission electron microscopy and X-ray photoelectron energy spectroscopy, respectively. Both solid and hollow CoO nanospheres as anode for lithium-ion battery were tested by galvanostatic discharge–charge experiments. The first discharge capacity of 1598 and 1640 mAh g−1 was obtained at 0.1C for solid and hollow CoO nanospheres, respectively. Hollow CoO nanospheres showed better cycle performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since Poizot et al. [1] reported that transition-metal oxides (MO, where M is Co, Ni, Cu, or Fe) possess electrochemical properties of insertion and extraction of lithium-ions, many researchers have conducted intensive investigations. Though CoO anode for lithium-ion battery exhibits high theoretical capacity of 715 mAh g−1 and good reversibility of electrochemical reaction, relevant works are much less than those of other MO [2]. We believed this is due to its rather complicated methods of synthesis which hindered its practical development and application as we known Co(II) can be either oxidized or reduced to Co(III) or Co. Seo et al. [3] have reported that CoO nanocrystals can be prepared by thermal decomposition of Co(acac)3 (acac = acetylacetonate) under Ar atmosphere. Zhang et al. [4] reported that CoO nanocrystals can be synthesized by annealing Co(II) oleate under N2 flow. Yao et al. [5] have synthesized CoO platelets by synthesizing precursor Co(OH)2 followed by annealing in flowing Ar. In addition to be used as anodic material for lithium-ion batteries [6], CoO has potential applications such as magnetic data storage devices [7], catalysis [8], etc. To seek simple and cost effective synthetic method is still remained as one of the major challenges up to date.
Solvothermal synthesis is an effective method to prepare novel materials under mild temperature and high pressure in sealed apparatus. Due to availability of large variety of solvents and surfactants, solvothermal synthesis has been intensively investigated to prepare materials with different sizes and morphology. Song et al. [9] reported synthesis of CeO2 hollow spheres via oleic acid assisted solvothermal method. Gao et al. [10] successfully synthesized monodisperse Fe3O4 nanoparticles by solvothermal route. Oleic acid as a surfactant and structure-directing agent has been widely used for synthesis of nanomaterials. Herein, synthesis of both solid and hollow CoO nanospheres of high purity have been developed by solvothermal method with oleic acid as reactant and SiO2 as template. Solid CoO nanospheres with diameters of 100–300 nm and hollow CoO nanospheres with diameters of 350–550 nm have been successfully synthesized. In addition to reactions occurred in synthetic process being depicted in detail, formation mechanisms of relevant have been proposed. The electrochemical properties of both solid and hollow CoO nanospheres as anodic materials for lithium-ion battery were determined by galvanostatic discharge–charge tests.
Experimental section
Synthesis of solid and hollow CoO nanospheres
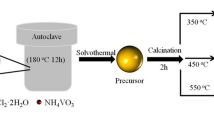
All chemical reagents used in this study were of analytical grade and used with no modification. 0.85 g Co(NO3)2·6H2O was dispersed in ethanol absolute and 10 mL oleic acid was dripped into the solution with vigorous stirring. The mixture was transferred to 50 mL Teflon-lined stainless steel autoclave, which was filled with ethanol absolute up to 80 % of its total volume. The autoclave was sealed and heated at 140 °C for 10 h in an electrical oven. After heating treatment, the autoclave was cooled to room temperature. Black product was obtained, washed with ethanol absolute and distilled water several times and dried in an electrical oven at 80 °C.
SiO2 nanospheres were prepared by Stober’s method [11]. 1.0 g Co(NO3)2·6H2O was dispersed in 30 mL ethanol absolute to make the precursor, followed by the addition of 10 mL oleic acid and 1.0 g SiO2 nanospheres into the solution. After vigorous stirring for 30 min, the solution was transferred to 50 mL Teflon-lined stainless steel autoclave. After heating treatment at 140 °C for 10 h in an electrical oven, the autoclave was cooled to room temperature to get CoO covered SiO2 composite nanospheres. The composite was first washed with ethanol absolute and distilled water for four times. Finally, hollow CoO nanospheres were obtained by removing SiO2 core or template in 2 M NaOH solution for 24 h and washed with distilled water for three times.
Characterization of solid and hollow CoO nanospheres
The crystal phases and chemical compositions of the as-synthesized samples were characterized by Rigaku D/max-2500 type X-ray diffraction (XRD) apparatus with Cu-Kα radiation (λ = 1.5406 Å). Morphology of the samples was observed by field-emission scanning electron microscopy (SEM, Siron 200) and transmission electron microscopy (TEM, Philips Tecnai 20). The sample composition and oxidation state of element were analyzed by X-ray photoelectron spectra (XPS, K-Alpha).
Electrochemical performance measurements
Electrochemical property was characterized by CR2016 type coin cells. The active material (solid or hollow CoO nanospheres), conductivity agent (carbon black) and binder (polyvinylidene difluoride) were mixed at weight ratio of 80:10:10 in a solvent (N-methyl-2-pyrrolidone). Methods of cell preparation were introduced in our previous paper [12]. Galvanostatic discharge and charge tests were carried out on LAND CT2001A system.
Results and discussion
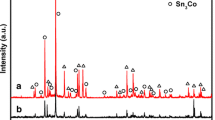
XRD patterns of solid and hollow CoO nanospheres are shown in Fig. 1. All diffraction peaks of solid and hollow CoO nanospheres at (111), (200), (220), (311), and (222) are in excellent accordance with corresponding standard cubic CoO phase (JCPDS, 48-1719) belonging to space group Fm3hm (a = b = c = 4.260 Å). Mean grain size (D) of solid CoO nanospheres was calculated by Scherrer formula according to (200) plane of 21.3 nm. No other peaks of impurities have been detected, indicating good crystallinity.
SEM images of solid and hollow CoO nanospheres have been shown in Fig. 2. Figure 2a shows that solid CoO sample consists of numerous nanospheres. Diameters of solid CoO nanospheres are in the range 100–300 nm. Figure 2b shows that diameters of well-dispersed SiO2 nanospheres are in the range 300–500 nm. The composite nanospheres are presented in Fig. 2c. Note, these composite nanospheres are much rougher than those of SiO2 with certain degree of agglomeration. Hollow CoO nanospheres are shown in Fig. 2d after removing of SiO2 core or template. Note, some hollow spheres have been broken and most of them formed agglomeration. To further observe their microstructures, TEM images of solid and hollow CoO nanospheres are presented in Fig. 2e, f. From Fig. 2e, we can see there are numerous solid nanospheres with diameters of above 200 nm presented without holes inside. On the other hand, Fig. 2f reveals the shell thickness of hollow CoO nanospheres is around 50 nm with diameters in the range 350–550 nm.
To our knowledge, simple shaped CoO including nanocrystal [4], nanowires [13], nanorod [14], and hollow spheres [15] has been produced up to now. Here, we have demonstrated the capability of synthesizing both solid and hollow CoO nanospheres through solvothermal route.
We have employed ethanol absolute as the solvent in reaction system. Oleic acid can be ionized in ethanol absolute to coordinate Co2+ provided by Co(NO3)2 to form \( {\text{Co}}[{\text{CH}}_{ 3} ({\text{CH}}_{2} )_{7} {\text{CH = CH}}({\text{CH}}_{2} )_{7} {\text{COO}}]_{2} \) complex [16, 17]. In the end, CoO nanospheres were synthesized by esterification reaction of \( {\text{Co}}[{\text{CH}}_{3} ({\text{CH}}_{2} )_{7} {\text{CH = CH}}({\text{CH}}_{2} )_{7} {\text{COO}}]_{2} \) and ethanol under solvothermal condition. These reactions can be described as follows [18]
High-purity solid and hollow CoO nanospheres have been characterized by XRD, FESEM, TEM, and XPS, respectively. Besides, we were able to avoid impurity phase introduced by incomplete protective atmosphere and decomposition.
Formations of solid and hollow CoO nanospheres are proposed in Fig. 3. Oleic acid can coordinate Co2+ to form complex, and then produce a great deal of micelles due to collision of these complexes and interaction of long alkyl chains in oleic acid [16]. Oleic acid provides reaction sites and takes part in formation reaction of CoO nanospheres under solvothermal condition [19]. Therefore, oleic acid played double roles of reactant and structure-directing agent in the system. According to the well-known Gibbs–Thomson law, these micelles further grow into spheroids. After reaction, black product was washed with ethanol absolute and distilled water several times to obtain pure solid CoO nanospheres (see Fig. 3a. Formation process of hollow CoO nanospheres is proposed in Fig. 3b. SiO2 was used as a soft template in solution and CoO particles that were synthesized in reaction system were coated on surfaces of SiO2 nanospheres. Shell thickness of CoO about 50 nm (see Fig. 2f) and core SiO2 composite nanospheres were prepared. Finally, SiO2 template was removed in 2 M NaOH solution and hollow CoO nanospheres have been obtained.
To further analyze composition and oxidation state of as-prepared samples, X-ray photoelectron energy spectroscopy (XPS) was recorded in K-Alpha instrument using Al-Kα radiation source. Figure 4a is full survey XPS spectrum of CoO, which has been indexed to C1s, O1s, and Co2p regions. There is a small quantity of C1s peak, which is useful for energy calibration. Figure 4b is a magnified region of Co2p core level. The peaks at 780.1 and 796.5 eV belong to Co2p3/2 and Co2p1/2, respectively. Two shake-up satellite peaks appeared at 785.7 and 802.3 eV, respectively. The presence of those two peaks and corresponding satellite peaks nearby are consistent with the presence of Co(II) in high-spin state in cubic CoO [13, 20]. There is no feature peak of Co metal at 778.1 eV. XPS is a reliable technique for intensive study of nano-scale materials, especially efficient for investigating the statues of atoms deep into a few layers on surface of nano-scale materials. Therefore, based on XPS analysis and XRD data, the sample synthesized here has to be pure CoO.
Discharge and charge profiles of solid and hollow CoO nanospheres at 0.1C (71.5 mA g−1) in the potential range of 0–2.5 V are shown in Fig. 5. The first discharge capacity and charge capacity of solid CoO nanospheres are 1598 and 905 mAh g−1, respectively. Hollow CoO nanospheres exhibit first discharge capacity and charge capacity of 1640 and 954 mAh g−1, respectively. Both charge capacities are higher than its theoretical capacity of 715 mAh g−1 reported in the literatures [2, 6]. Yao et al. [5] reported that first discharge capacity of CoO platelets was 1107 mAh g−1 at current rate of 100 mA g−1. Do and Weng [21] reported that decreasing the grain and particle sizes of CoO can increase its discharge capacity. Chen confirmed that the amount of anomalous capacity is related to nano-sized CoO sample [2]. Solid and hollow CoO nanospheres possess large surface area with more reaction sites on surface must be responsible for our results. Irreversible capacity for the first cycle derived from formation of solid electrolyte interface (SEI), decomposition of electrolyte and other factors. There is a long voltage plateau occurred at about 0.75 V in first discharge curve, ascribed to reduction of CoO to metallic Co (see Eq. 4). The voltage plateau appeared between 1.2 and 1.6 V in first charge curve, derived from oxidation of nano-sized Co particles with Li2O (Eq. 4). Electrochemical reactions of solid and hollow CoO nanospheres may be described as follows, based on experimental phenomenon and literatures [1, 2]
As shown in Fig. 6, discharge capacity of solid CoO nanospheres is 452 mAh g−1 at 0.1C after 50 cycles. Discharge capacity of hollow CoO nanospheres is 832 mAh g−1 at the same condition, which is 1.84 times higher than that of solid CoO nanospheres. Hollow CoO nanospheres showed better reversible cycle performance. Literatures have reported that hollow structural materials may improve anode cycle performance [22, 23]. The excellent electrochemical performance should be related to this fact. On one hand, hollow CoO structure alleviates/reduces the volume change during cycling for lithium-ions insertion and extraction. On the other hand, thin CoO shell reduced the diffusion route of the lithium-ion.
Conclusions
In summary, high-purity solid and hollow CoO nanospheres have been synthesized at mild temperature by solvothermal method. Formation processes of solid and hollow CoO nanospheres have been proposed in detail. The hollow CoO nanospheres exhibit excellent electrochemical property of high discharge capacity and possess potential use as anode material for next-generation lithium-ion battery.
References
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon J (2000) Nature 407:496
Chen CH, Hwang BJ, Do JS, Weng JH, Venkateswarlu M, Cheng MY, Santhanam R, Ragavendran K, Lee JF, Chen JM, Liu DG (2010) Electrochem Commun 12:496
Seo WS, Shim JH, Oh SJ, Lee EK, Hur NH, Park JT (2005) J Am Chem Soc 127:6188
Zhang Y, Zhu J, Song X, Zhong X (2008) J Phys Chem C 112:5322
Yao W, Yang J, Wang J, Nuli Y (2008) J Electrochem Soc 155:A903
Wu Z, Qin L, Pan Q (2011) J Alloys Compd 509:9207
Motohashi K, Ikeda N, Sato T, Shiga D, Ono H, Onodera S (2008) J Magn Magn Mater 320:3004
Moussa S, Abdelsayed V, El-Shall MS (2011) Chem Phys Lett 510:179
Song Y, Wei J, Yang Y, Yang Z, Yang H (2010) J Mater Sci 45:4158. doi:10.1007/s10853-010-4505-5
Gao G, Shi R, Qin W, Shi Y, Xu G, Qiu G, Liu X (2010) J Mater Sci 45:3483. doi:10.1007/s10853-010-4378-7
Stober W, Fink A, Bohn E (1968) J Colloid Interface Sci 26:62
Wen Z, Zheng F, Liu K, (2012) Mater Lett 68:469
Wang W, Zhang G (2009) J Cryst Growth 311:4275
An K, Lee N, Park J, Kim S, Hwang Y, Park J et al (2006) J Am Chem Soc 128:9753
Shi R, Chen G, Ma W, Zhang D, Qiu G, Liu X (2012) Dalton Trans 41:5981
Teng F, Yao W, Zhu Y, Gao G, Meng DD (2009) J Non Cryst Solids 355:2375
Wang X, Zhuang J, Peng Q, Li Y (2005) Nature 437:121
Ye Y, Yuan F, Li S, (2006) Mater Lett 60:3175
Sun G, Zhang X, Cao M, Wei B, Hu C (2009) J Phys Chem C 113:6948
Lagunas A, Payeras AM, Jimeno C, Puntes VF, Peric MA (2008) Chem Mater 20:92
Do J, Weng C (2005) J Power Sources 146:482
Lou X, Wang Y, Yuan C, Lee JY, Archer L (2006) Adv Mater 18:2325
Han S, Jang B, Kim T, Oh S, Hyeon T (2005) Adv Funct Mater 15:1845
Acknowledgements
This study was supported by State Key Laboratory of Powder Metallurgy of China, and the Science and Technology Foundation of Guizhou Province, China (Grant No. [2011]2030).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wen, Z., Zheng, F., Jiang, Z. et al. Solvothermal synthesis of solid and hollow CoO nanospheres and their electrochemical properties in lithium-ion battery. J Mater Sci 48, 342–347 (2013). https://doi.org/10.1007/s10853-012-6751-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6751-1