Abstract
The influence of Mn content on the wettability of dual phase high strength steels (1.8, 2.2, and 2.6 wt% Mn at a fixed Si content of 0.12 wt%) was investigated by a dispensed sessile drop method with liquid Zn—0.23 wt% Al at 450 °C (723 K). Before the wetting tests, the samples were annealed in a galvanizing simulator under 15 % H2–N2 atmosphere, and the surfaces were analyzed by scanning electron microscopy, Fourier transform infrared spectroscopy, and a transmission electron microscope. It was found that the surface coverage of the oxides for the three samples at different Mn contents of 1.8, 2.2, and 2.6 wt% were estimated to be 0.55, 0.58, and 0.71, respectively, and surface oxide modification occurred from MnSiO3 to Mn2SiO4 to MnO as the Mn content increased. From the wetting experiments, the initial contact angle increased from 101 to 110°, which could be explained by classical wetting theory by means of the Cassie equation. On the other hand, the reactive wetting was affected by the sorts of surface oxides and the fraction. It was considered that the dissolved Al reduced MnO to increase the bare metallic Fe surface at the triple line, which enabled the continuing reactive wetting process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dual-phase (DP) high-strength steels have been utilized as automotive structural materials due to their combination of excellent ductility and high strength [1]. DP steels typically contain Si and Mn to facilitate martensitic transformation and to prevent bainite or pearlite formations, respectively. However, Mn and Si are preferentially oxidized to form nanometer-sized Mn x Si y O z oxides during the annealing process before hot-dip galvanizing [2–4]. These oxides show poor wettability by liquid zinc alloys [5], resulting in uncoated bare spots on the steel surface after hot-dip galvanizing [3, 5]. From the microscopic investigations and bend tests, Khondker et al. [6] observed good reactive wetting for high-strength DP steel with 2 wt% Mn by liquid Zn—0.2 wt% Al alloy. They asserted that the aluminothermic reduction of the surface MnO by the dissolved Al in the Zn bath (Eq. (1)) could facilitate the reactive wetting.
Recently, we quantitatively investigated the wettability of DP high-strength steels by changing the Si concentration (0–1.0 wt%) with a fixed Mn content (1.8 wt%) [7, 8]. It was found that the surface oxides were modified from MnO to Mn2SiO4 to MnSiO3 to SiO2 as the Si content increased. The modification of the surface oxides yielded a change in the contact angle [7]. The samples with Si content lower than 0.2 wt% showed relatively good wettability by liquid Zn–Al alloy. It was noticeable that the sample of 0 wt% Si, which formed only MnO on the surface, showed the most dramatic change in the contact angle during the galvanizing process time (5 s). After hot-dip galvanizing, a thin and uniform Fe2Al5Zn x intermetallic compound layer (the so-called inhibition layer) was formed when the Si content was lower than 0.2 wt% [8]. However, as the Si content increased further, the inhibition layer became thicker and non-uniform, and eventually uncoated bare spots were found after hot-dip galvanizing [8].
The purpose of this study is to find a steel composition for better wetting properties by the modification of surface oxides. In order to investigate the influence of the surface oxide modification to MnO, the Mn content in the steel was varied to be 1.8, 2.2, and 2.6 wt% Mn. Since Si is essential to facilitate the martensitic transformation of the steel in an industrial process, the Si content was fixed at 0.12 wt%.
Experimental
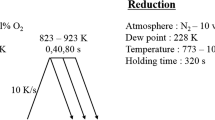
Table 1 shows the chemical analysis results of the steel samples used in this study. Each sample was annealed at 800 °C (1,073 K) for 40 s in a reducing atmosphere of 15 %H2–N2 at a dew point temperature of −60 °C (213 K). After annealing, the surface oxides were analyzed. The morphology of the surface oxides was investigated by scanning electron microscopy (SEM, JEOL, JSM-6700F). The type of the surface oxides was investigated by Fourier transform infrared spectroscopy (FT-IR, Shimadzu, IRPrestige-21) and a transmission electron microscope (TEM, JEOL/JEM-2100). TEM samples were prepared with a focused ion beam (FIB, FEI, Nova200), and the cross sections of the samples were investigated. Zn—0.23 wt% Al alloy was prepared in an induction furnace under a purified Ar atmosphere. The Al concentration in the zinc alloy was analyzed with inductively coupled plasma atomic emission spectroscopy (ICP-AES, Varian, Vista-PRO). For the wetting experiments, a lab-made dispensed drop test machine was used. Details of the wetting experiment procedures are described in [9].
Results
SEM analyses
Figure 1 shows the SEM images of the steel surface. The morphology of the nanometer-sized surface oxides did not change so much with increasing Mn content. Since the surface oxides were small spherical particles, it was possible to distinguish the surface oxides from the bare metal surface and determine the coverage of the surface oxides. From the SEM image analysis, the coverage of the surface oxides for the three samples at different Mn contents of 1.8, 2.2, and 2.6 wt% were estimated to be 0.55, 0.58, and 0.71, respectively. Therefore, it was concluded that the coverage of the surface oxides increased by increasing Mn content.
Effect of Mn concentration on the surface oxides morphology of DP high-strength steels: a 1.8 wt% Mn [3], b 2.2 wt% Mn, c 2.6 wt% Mn
FT-IR and TEM analyses
Figure 2 shows the FT-IR results of the surface oxides. For the sample of 1.8 wt% Mn, most the surface oxides were identified as Mn2SiO4. On the surfaces of the 2.2 and 2.6 wt% Mn samples, the MnO peak heights were increased as the Mn content increased, while Mn2SiO4 was a dominant surface oxide. Figure 3 shows the TEM dark field images of the cross sections of the samples. TEM analysis results were in reasonable accordance with the FT-IR analysis results. From the energy dispersive spectrometer (EDS) analyses, the surface oxides on the 1.8 wt% Mn sample were identified as Mn2SiO4 and MnSiO3. On the surface of the 2.2 wt% Mn sample, MnO was found together with Mn2SiO4 and MnSiO3. For the 2.6 wt% Mn sample, MnO was found on the surface as well as in the bulk (internal oxidation). Therefore, it was concluded that the surface oxide was modified from MnSiO3 to Mn2SiO4 to MnO, as the Mn content increased.
Characteristic FT-IR spectra according to Mn contents in DP high-strength steels. The result of 1.8 wt% Mn is from [8]
Contact angle versus time
Figure 4a shows the change of the contact angle from 0 to 0.1 s. It was found that in this initial period, the oscillation of the contact angle was considerable, but diminished for t > 1 s. Similar results were investigated in previous studies [6, 9–11]. During the initial 0.1 s period, the contact angle varied between the advancing and receding angles. Shiraz et al. investigated a similar initial oscillation of the contact angle of a liquid tin droplet on steel substrates at 513 K (240 °C) [12]. They reported that the contact angle varied between 110 and 160° when the impact velocity of the liquid droplet on the substrate was 1.0 m/s, while the contact angle varied between 100 and 140° when the impact velocity was 3.0 m/s. Therefore, the initial oscillation can be affected by the external forces acting at the moment of the drop impact. On the other hand, Kim et al. [13] reported that the initial contact angle can also be oscillated due to the competition between the movement of the gas–liquid–solid triple line and the growth of a new intermetallic compound at the triple line. At the moment, it is not clear which factor is dominant in the present study. Nevertheless, the mean values of the advancing and the receding angles were almost constant in the initial period. Therefore, the mean value could be assumed as a quasi-equilibrium angle characterizing wetting of the initial period. Figure 4b shows the change of the contact angle from 0 to 10 s. The galvanizing process takes approximately 5 s or less from the starting point of the hot dipping to the solidification. Therefore, it is considered that the contact angle at 5 s can be treated as a comparable wetting index of steels in the galvanizing process.
Figure 5 shows the effect of Mn content on the contact angle at 0 and 5 s. The initial contact angle (the quasi-equilibrium angle at t ≈ 0 s) for the three samples at different Mn contents of 1.8, 2.2, and 2.6 wt% were estimated to be 101, 101, and 110°, respectively. Accordingly, the 2.6 wt% Mn steel showed the worst initial wettability. As the reactive wetting proceeded between the liquid zinc alloy and steel plates, the contact angle decreased. The contact angle at 5 s for the three samples at different Mn contents of 1.8, 2.2, and 2.6 wt% were estimated to be 80, 75, and 78°, respectively. Consequently, the 2.2 wt% Mn sample showed the best wettability.
Effect of Mn contents on the contact angles of DP high-strength steel substrates by liquid Zn—0.23 wt% Al alloy. The result of 1.8 wt% Mn is from [7]
Discussion
Initial wetting
According to traditional wetting theory, the initial wetting behavior of liquid on a metal-oxide mosaic surface can be explained by the Cassie Eq. (2) [14].
where \( \theta_{c} \) is the Cassie contact angle, \( \theta_{Fe} \) is the equilibrium contact angle on the Fe surface, \( \theta_{o} \) is the equilibrium contact angle on the oxide surface, and \( f_{o} \) is the coverage of the surface oxides. Kawano and Ronner [15] verified that the Cassie equation was valid for the initial wetting of liquid Zn on patterned Fe–Al2O3 mosaic surfaces. Many researchers have tried to use Eq. (1) in the analyses of the initial wetting of liquid Zn and Zn alloys on complex mosaic steel surfaces with nanometer-sized oxides [6, 9, 11, 16]. Since the surface oxides were composed of MnO, Mn2SiO4, and MnSiO3, the equilibrium contact angle of the oxide surface can be treated as a mean value of these oxides. Figure 6 shows the cosine of the contact angles as a function of \( f_{o} \). For comparison, the cosine of the contact angles of liquid Zn—0.19 wt% Al on pure iron [17] and the cosine of the contact angle of liquid Zn—0.23 wt% Al on solid oxides [5] are plotted together. The present experimental results show reasonable accordance with the line predicted by the Cassie equation.
Reactive wetting
As the reactive wetting proceeded, the contact angle gradually decreased, which can be related to the formation of Fe2Al5Zn x intermetallic compound layer between liquid Zn alloy and the steel substrate [18, 19]. This new phase might prevent or decrease the direct contact between the liquid Zn alloy and the surface oxides and eventually decreased the interfacial tension.
It is noteworthy that the reactive wetting behaviors of the 1.8 and 2.2 wt% Mn samples are slightly different, even though the initial contact angle and the coverage of the surface oxides are almost the same. The contact angle of the 2.2 wt% Mn sample at t = 5 s was lower than that of the 1.8 wt% Mn sample. Frenznick et al. [16] reported that the reactive wetting depends on the growth of a stable inhibition layer. Kawano and Renner [15] confirmed that the triple line continuously moves to form an inhibition layer between metallic Fe and soluble Al in the liquid Zn alloy. When MnO meets the liquid Zn–Al alloy at the triple line on the DP high strength steel surface, it can be reduced by the dissolved Al in the liquid Zn alloy [6]. Alibeigi et al. [20] asserted that the aluminothermic reduction of MnO and the exposure of the underlying metallic Fe occur at the triple line, and behind the triple line, the inhibition layer forms. Direct observation of the reduction of MnO on an oxidized Si wafer by dissolved Al in liquid Zn alloy was carried out by Kawano and Renner [21]. They reported that the decrement of the MnO by the reaction was estimated to be about 3-nm thick after 7.4 s. Therefore, it is speculated that the reduction of MnO partially occurred. A schematic illustration of the reactive wetting is given in Fig. 7. As the triple line moves continuously, the MnO at the triple line can be partially reduced by the dissolved Al, exposing new surfaces to the liquid Zn. Consequently, the reactive wetting process can be facilitated by increasing the MnO fraction at the same initial coverage of the surface oxides.
When the reactive wetting of the 2.2 and 2.6 wt% Mn samples are compared, not only the initial contact angle but also the contact angle at t = 5 s of the 2.2 wt% Mn sample is lower than that of the 2.6 wt% sample. Although the 2.6 wt% Mn sample would have more MnO particles on the surface than the 2.2 wt% Mn sample, the initial coverage of the surface oxides was much higher. Therefore, it is concluded that the reduction of MnO and the increase of the bare metal surface area were not large enough to overcome the difference in the initial surface coverage of the oxides of these samples.
Conclusion
In this study, the influence of Mn concentration on the wettability of DP high strength steels by liquid Zn—0.23 % Al was investigated. It was found that the wettability in the initial stage of wetting was mostly affected by the surface coverage of oxides and the experimental results could be explained by the Cassie equation. However, as the reactive wetting proceeded, the contact angle was affected by the type of surface oxides and the fraction. The formation of MnO slightly improved the reactive wetting behavior due to the partial reduction of MnO by the dissolved Al.
References
Mintz B (2001) Int Mater Rev 46:169
Suzuki Y, Yamashita T, Sugimoto Y, Fujita S, Yamaguchi S (2009) ISIJ Int 49:564
Jeon S, Chin K, Shin K, Sohn H, Kim D (2008) J Kor Inst Met Mater 46:289
Jeon S, Shin K, Sohn H, Kim D (2008) J Kor Inst Met Mater 46:427
Kim Y, Shin M, Tang C, Lee J (2010) Metall Mater Trans B 40B:872
Khondker R, Mertens A, McDermid JR (2007) Mater Sci Eng A 463:157
Kim Y, Lee J, Park J, Jeon S (2011) Metall Mater Int 17:607
Jeon S, Chin K, Shin K, Lee J, Sohn H (2009) J Kor Inst Met Mater 47:423
Lee J, Park J, Jeon SH (2009) Metall Mater Trans B 40B:1035
Lee J, Park J, Kim Y, Jeoun SH (2010) J Mater Sci 45:2112. doi:10.1007/s10853-009-4131-2
Lee J, Park, Jeon SH (2011) Metall Mater Trans B 42B:1086
Aziz SD, Chandra S (2000) Int J Heat Mass Trans 43:2841
Kim T, Lee J, Kim Y, Kim J-M, Yuan Z (2009) Mater Trans 50:2695
Cassie A (1948) Discuss Faraday Soc 3:11
Kawano T, Renner F (2011) ISIJ Int 51:1703
Frenznick S, Swaminathan S, Stratmann M, Rohwerder M (2010) J Mater Sci 45:2106. doi:10.1007/s10853-009-4147-7
Hara S, Asano H (1992) CAMP-ISIJ 5:1737
Frenznick S, Stratmann M, Rohwerder M (2008) Rev Sci Instr 79:043901
Giorgi M, Guillot J (2005) J Mater Sci 40:2263. doi:10.1007/s10853-005-1944-5
Alibeigi S, Kavitha R, Meguerian RJ, McDermid JR (2011) Acta Mater 59:3537
Kawano T, Renner F (2012) Surf Interface Anal. doi:10.1002/sia.4928
Acknowledgements
This project was supported by a grant from Korea University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y., Lee, J., Jeon, SH. et al. The influence of Mn Content on the wettability of dual-phase high-strength steels by liquid Zn—0.23 % Al. J Mater Sci 47, 8477–8482 (2012). https://doi.org/10.1007/s10853-012-6737-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6737-z