Abstract
Zinc aluminate co-doped with Yb3+/Er3+ for potential application in upconversion lasers was prepared in proportions of 2:1, 5:1, and 10:1 by combustion reaction. The samples were characterized by X-ray diffraction, transmission electron microscopy, and optical spectroscopy. The results reveal the formation of crystalline ZnAl2O4 primary phase and trace amounts of ZnO and Yb3Al5O12 secondary phases. They also indicate that increasing the Yb3+/Er3+ ratio favored the increase of secondary phases. The optical spectroscopy analysis revealed that red emission predominated over green, and that emission intensity was directly influenced by the infrared intensity of the diode laser. These results indicate that ZnAl2O4 doped with rare earth ions may also be an interesting material for luminescence obtained by energy upconversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Semiconductor nanocrystals doped with rare earth ions have been investigated extensively in recent years. These materials show interesting enhanced optical properties with potential applications in the design of optoelectronic materials and as efficient phosphor materials for flat-panel displays [1, 2]. This semiconductor matrix co-doped and/or doped with rare earth ions has become increasingly interesting for these technological applications operating in the visible infrared (IR)-excited band. To date, a variety of semiconductor compositions co-doped with Er3+ e Yb3+ ions have been investigated for the possible application of their optical properties in telecommunications, e.g., in optical amplifiers operating by energy transfer (ET) upconversion [3]. Emission mechanisms have been investigated exhaustively in several hosts in different ceramic matrices [4, 5].
Some of the most relevant works pertaining to this field of investigation are studies on ET and upconversion luminescence in Er/Yb co-doped transparent glass–ceramics containing YF3 nanocrystals [6]; the upconversion luminescence properties of LaF3:Yb3+, Er3+ nanocrystals, and investigations into the effect of excitation power density on the upconversion luminescence of LaF3:Yb3+, Er3+ nanocrystals [7]; the Judd–Ofelt analyses and luminescence of Er3+/Yb3+ co-doped transparent glass–ceramics containing NaYF4 nanocrystals reported by Ping et al. [8]; the preparation and spectroscopic properties of nanostructured glass–ceramics containing Yb3+, Er3+ ions and Co2+-doped MgAl2O4 nanocrystals described by Chen et al. [9]; and You et al.’s [10] study of the upconversion properties of Yb3+ and Er3+ doped Y4Al2O9 phosphors.
Among these various types of matrices, aluminum-based spinels are an interesting type of oxide ceramics with significant technological applications, a fact that has motivated several research groups to focus their efforts on the synthesis and characterization of rare earth-doped ZnAl2O4 phosphors. Some of the most relevant papers on this subject include ultrafine gahnite (ZnAl2O4) nanocrystals: hydrothermal synthesis and photoluminescent properties reported by Chen et al. [11]; the work of Menon et al. [12], who evaluated the thermally stimulated luminescence and optically stimulated luminescence properties of Tb-doped ZnAl2O4 prepared by combustion synthesis; the preparation of spherical porous ZnAl2O4:Eu3+ phosphors: PEG-assisted hydrothermal growth and photoluminescence reported by Chen and Ma [13]; and a study of the optical properties of ZnAl2O4:Eu3+ hollow nanophosphors by Barros et al. [14].
Gahnite (ZnAl2O4) is a useful semiconductor (3.8 eV) which, in its polycrystalline form, is transparent to visible light at wavelengths of >320 nm. This makes it suitable for use as a transparent conductor and as a dielectric, optical [15], and catalytic material [16]. ZnAl2O4 has attracted much interest for applications in various fields, including reflective optical coatings in aerospace applications, as a phosphor material, and as an ultraviolet-transport electroconductive oxide [17].
Rare earth dopants, which normally exist in the form of trivalent ions, show very narrow line fluorescence bands and exhibit a characteristic intra 4f shell luminescence, which is nearly independent of both host material and temperature [18]. ZnAl2O4 phosphors activated by rare earth metal ions have been studied due to the unique luminescent properties resulting from their high thermal and chemical stability and high emission quantum yields [13] showing a promising potential for use as a host lattice for rare earth ions.
In this context, this study investigated the upconversion luminescence of ZnAl2O4 co-doped with Yb+3/Er3+ ions in proportions of 2:1, 5:1, and 10:1 mol, prepared by combustion reaction, for potential application in lasers.
Experimental
The precursor materials used in this work were zinc nitrate Zn(NO3)2·6H2O (Merck), aluminum nitrate Al(NO3)3·9H2O (Aldrich), and Er2O3 and Yb2O3 rare earth oxides (Aldrich), which were used as oxidizers and a source of metallic cations. Urea [CO(NH2)2] was used as a reducing agent. All the reagents used here were of analytical grade with 98–99.9 % purity. The initial composition of the solution containing the nitrates, oxides, and urea was based on the total valence of the oxidizing reagents and reducing agent, based on the concept of propellant chemistry [19, 20], which considers carbon (+4), hydrogen (+1), aluminum (+3), zinc (+2), ytterbium, and erbium (+3) as reducing elements. Oxygen was considered an oxidizing element with a valence of (−2) and the valence of nitrogen was considered zero because it is inert. The Yb3+/Er3+ reagents used for doping the ZnAl2O4 matrix in ratios of 2:1, 5:1, and 10:1 were weighed and placed in a Pyrex beaker to which 5 mL of distilled water was added to form stoichiometric solutions. These solutions were then heated on a hot plate at 480 °C until they self-ignited. The products of the combustion reaction were then placed in a muffle furnace preheated to 500 °C where they were left for about 15 min to eliminate any remaining volatile compounds (from urea and nitrate decomposition) that might still be present due to the short time of reaction. The resulting porous flake-shaped powders were disagglomerated in a mortar agate, sifted through a 325 mesh (44 μm) sieve, and then characterized. The reaction temperature was measured with a RAYTEK RAYCI IR pyrometer, while the flame combustion temperature was measured with a RAYTEK MA2SC IR pyrometer, and the flame time with a CONDOR digital chronometer. The reactions’ explosiveness and flame color were evaluated visually during the experiment.
The samples were characterized by X-ray diffraction (XRD, SHIMADZU 6000, equipped with a Ni filter, using Cu Kα radiation, a scan rate of 2° 2θ/min, in a 2θ range of 10–75°, voltage of 40 mA, in steps of 0.02°, with a step time of 1 s, at room temperature—25 °C). By means of Scherrer’s equation [21], the crystallite size was calculated from the broadest peaks of the most intense XRD lines of ZnAl2O4, which were d(311) d(220) d(440) d(511). The morphology of the samples was then examined by transmission electron microscopy (TEM) (Phillips EM420), operating at 120 kV). The entire spectroscopic characterization of the samples was performed at room temperature. Ground-state absorption (GSA) spectra were measured using a Lambda 900 spectrophotometer (Perkin Elmer) operating in the range of 700–1,700 nm. For these measurements, the samples were ground into powder and pressed into KBr pellets. The upconversion spectra were recorded at 980 nm using a homemade diode laser as the excitation source. The powder samples were placed in capillary tubes and their luminescent signals were filtered using a single monochromator (0.3 m) (Thermo Jarrell Ash 82497). The filtered signals were collected with a photomultiplier (RCA 31034) and amplified with a lock-in amplifier (PAR 128).
Results
Table 1 lists the combustion flame time, combustion flame temperature, and flame color of the reactions. As can be seen, all the samples presented a yellow flame color. The average plate temperature was 531 °C, which is slightly higher than the maximum temperature of 480 °C recommended by the manufacturer. The slight increase in combustion flame time and temperature was due to the increase in the Yb3+/Er3+ ratio.
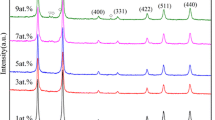
Figure 1 shows the XRD patterns of the ZnAl2O4:Yb3+/Er3+ samples prepared by combustion reaction and containing Yb3+/Er3+ ratios of 2:1, 5:1, and 10:1. These patterns indicate the formation of primary crystalline product ZnAl2O4 spinel (JCPDS #82-1136) and traces of secondary product ZnO (gahnite) (JCPDS #75-0576) in all the samples. The 10:1 sample also showed the presence of Yb3Al5O12 (JCPDS #23-1476) product. The calculated crystallite sizes of the primary phase were 14, 16, and 11 nm in the 2:1, 5:1, and 10:1 compositions, respectively. The results indicate a slight decrease in crystallite size with increasing amounts of Yb3+/Er3+. This may have been due to the presence of secondary product, which induced a more marked broadening of the peak and reduction in diffraction peak intensity.
The ionic radius of Yb3+ (0.75 Å) and Er3+ (0.85 Å) ions differed from the ionic radius of Al3+ (0.88 Å) ions by 15 and 3 %, respectively. This indicates that Al3+ ions are more easily replaced by Er3+ ions than by Yb3+ ions. Because the amount of Yb3+ used for co-doping was consistently higher than Er3+, and because its radius is 15 % lower than the ionic radius of Al3+, it is expected that increasing the amount of Yb3+ in co-doping (Yb3+/Er3+) favors a low solubility limit in the ZnAl2O4 lattice leading to the formation of secondary product rich in Yb3+ (Al3Yb3O12) as indicated in the XRD pattern.
The presence of primary product ZnAl2O4 and secondary product ZnO was also reported by Barros et al. [14] in their evaluation of the photophysical properties of Eu3+ and Tb3+-doped ZnAl2O4 phosphors obtained by combustion reaction. The authors stated that even with the presence of second phase, samples containing Eu3+ and Tb3+ ions displayed red and green photoluminescence, respectively.
Figure 2 quantifies the amount of products determined from the XRD data for the 2:1, 5:1, and 10:1 samples. As can be observed, the 2:1 sample showed the highest percentage of primary product ZnAl2O4 co-doped with Yb3+/Er3+ (85 %) and 15 % of secondary product ZnO. The 5:1 and 10:1 compositions showed a high percentage of primary product ZnAl2O4 co-doped with Yb3+/Er3+ and the presence of two secondary products. However, increasing the ratio of Yb3+ relative to Er3+ increased the percentage of secondary product ZnO, thereby reducing the concentration of the spinel phase.
Figure 3 shows bright field TEM micrographs of ZnAl2O4 co-doped with Yb3+/Er3+ in ratios of 2:1 and 5:1. Figure 3a (sample 2:1) reveals a highly homogeneous morphology of approximately hexagonal particles. In contrast, the morphology of sample 5:1 (Fig. 3b) is heterogeneous consisting of particles of different sizes and shapes, most of them approximately hexagonal. The average particle sizes of samples 2:1 and 5:1 are 7 and 28 nm, respectively. The particle size determined by TEM confirms the efficiency of the combustion synthesis reaction in producing nanoparticles, which tend to agglomerate due to their high reactivity (high surface energy).
Figure 4 shows the spectra of ZnAl2O4 co-doped with Yb3+/Er3+ in ratios of 2:1, 5:1, and 10:1 recorded using IR excitation wavelengths below 980 nm at room temperature. As can be observed, at wavelengths below 980 nm and near IR excitation, these samples show three well-defined, but low intensity, emission peaks at 525, 550, and 655 nm. The green emissions at 525 and 550 nm were attributed to 2H11/2 → 4I15/2 and 2S3/2 → 4I15/2 transitions, respectively, while the red emission at 655 nm was attributed to the 4F9/2 → 4I15/2 transition. The same behavior was reported by Qu et al. [7] in their study of the effect of excitation power density on the upconversion luminescence of LaF3:Yb3+, Er3+ nanocrystals. The 5:1 composition shows much more intense 4F9/2 → 4I15/2 (~655 nm) type transitions to red than the 2:1 composition, and very weak emission attributed to 2H11/2 → 4I15/2 (~525 nm), 2S3/2 → 4I15/2 (~550 nm) transitions to green. It was found that increasing the proportion of Yb3+ from 5:1 to 10:1 while maintaining a constant proportion of Er3+ (sample 10:1) gave rise to the transition 4F9/2 → 4I15/2, but with a lower intensity than in sample 5:1.
As Fig. 4 indicates, the emission spectrum of the 5:1 composition reveals transition characteristic of Er3+ ions centered at 655 nm (4F9/2 ↔ 4I15/2) and Yb3+ ions centered at 550 nm (2F5/2 ↔ 2F7/2). The 10:1 composition shows a decline in the intensity of luminescence resulting from the decrease in the distance between the dopant (activator) and the array, causing the migration of excitation energy among resonant ions of the same species, in this case Er3+ ions. This cross-relaxation (CR) mechanism causes a large amount of energy to dissipate in the form of non-radiative energy. It seems unlikely that CR had any effect at low concentrations in view of the large distance between actuators hindering the transfer of energy between them.
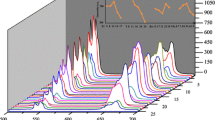
Figure 5 shows the room temperature upconversion spectra of the 5:1 composition in response to increasing excitation IR diode laser power. The spectrum of the conversion occurring in the region of 500–580 nm of the sample was visible at about 500–575 nm. It was found that when the excitation power density increased to a higher region, the intensities of green and red emission increased rapidly in response to increasing excitation power. Increasing the laser power augmented the temperature of the system causing the maximum emission bands at around 655 nm to increase and hence, increasingly favoring the 4F9/2 → 4I15/2 transitions.
In the frequency upconversion process, the upconversion emission intensity I up increases in proportion to the nth power of IR excitation intensity, I IR, i.e.,
where n is the number of IR photons absorbed per visible photon emitted. A plot of log I up versus log I IR yields a straight line with slope n. Figure 6 shows such a plot for the zinc aluminate co-doped with a 5:1 ratio of Yb3+/Er3+ under 980 nm excitation. At both emission intensities in the region of green and red wavelengths, note the linear dependence on increasing laser power showing slope coefficients of 2.6 and 1.94 obtained for green and red emissions, respectively. These results indicate that a two-photon process populates the 4S3/2, the 2H11/2, and the 4F9/2 levels. Similar results were reported by Sun et al. [22], who evaluated the optical transitions and frequency upconversion fluorescence of Er3+/Yb3+ co-doped strontium–lead–bismuth glasses. The authors observed intense green and red emissions centered at 525, 546, and 657 nm in response to 975 nm excitation, which corresponded, respectively, to transitions 2H11/2 → 4I15/2, 2S3/2 → 4I15/2, and 4F9/2 → 4I15/2, and reported that they obtained values of 1.87, 1.89, and 1.86 for n corresponding to the 525, 546, and 657 nm emission bands, respectively.
An analysis of the effect of co-doping with a 5:1 ratio of Yb3+/Er3+ ions indicated that the transition of 2H11/2 → 4I15/2 to green from the Yb3+ ions led to much lower upconversion intensity than that obtained with Er3+ ions in the transition of 4F9/2 → 4I15/2 to red. Therefore, the emission intensity of green luminescence does not exceed the red emission in the samples with a 5:1 ratio of Yb3+/Er3+.
As Fig. 6 indicates, the upconversion intensity of red emission showed a rapid growth on the upward line exceeding the green emission when co-doping with a 5:1 ratio of Yb3+/Er3+. This predominance of red emission over green emission was also observed by Wang et al. [23] in their study of the upconversion energy of Er3+ ions in ZnO nanocrystals produced by two-photon energy.
According to energy matching and the quadratic dependence on excitation power, the possible upconversion mechanisms for the emissions are discussed based on the simplified energy levels of Er3+ and Yb3+ presented in Fig. 7. For the green emissions, in the first step, the 4I11/2 level is directly excited with 980 nm light by GSA and/or by ET from the 2F5/2 level of Yb3+:2F5/2(Yb3+) + 4I15/2(Er3+) → 2F7/2(Yb3+) + 4I11/2(Er3+). Since the absorption cross section of Yb3+ is much larger than that of Er3+ in the 980 nm region, the ET mechanism predominates at the excitation level of 4I11/2. The second step involves excitation mechanisms based on the long-lived 4I11/2 level as follows: CR, 4I11/2(Er3+) + 4I11/2(Er3+) → 4F7/2(Er3+) + 4I15/2(Er3+); excited-state absorption (ESA), 4I11/2(Er3+) + a photon → 4F7/2(Er3+); and ET, 2F5/2(Yb3+) + 4I11/2(Er3+) → 2F7/2(Yb3+) + 4F7/2(Er3+). The populated Er3+ 4F7/2 level then relaxes rapidly and continues as follows: ET from Yb3+, 2F5/2(Yb3+) + 4I13/2(Er3+) → 2F7/2(Yb3+) + 4F9/2(Er3+); CR among Er3+ ions, 4I13/2 + 4I11/2 → 4I15/2 + 4F9/2; ESA, 4I13/2+ non-radiatively to the next lower levels 2H11/2; and 4S3/2 resulting from the small energy gap between them. Er3+ ions in the 2H11/2 state can also decay into the 4S3/2 state due to multiphonon relaxation.
Simplified diagram of the energy level of Er3+ and Yb3+ ions and possible transition pathways in zinc aluminate co-doped with Er3+/Yb3+ [10]
The estimated energy gap between the 2H11/2 state and the next lower state 4S3/2 is ~800 cm−1 [10]. Thus, the multiphoton relaxation rate is very high and the 525 nm emission intensity is reduced. These mechanisms then produce the two 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 green emissions centered at 525 and 546 nm, respectively. The red emission at 657 nm originates from the 4F9/2 → 4I15/2 transition and the population of 4F9/2 is based on the photon → 4F9/2. The 4I13/2 level is populated owing to non-radiative relaxation from the upper 4I11/2 level. In addition, the non-radiative process from 4S3/2 state, which is populated by means of the previously described process to the 4F9/2 level, also contributes to the red emission.
Conclusions
Synthesis by combustion reaction was favorable for obtaining ZnAl2O4 spinel: Yb3+/Er3+ with an average crystallite size of 11–16 nm and particle sizes ranging from 7 to 28 nm. IR-to-visible upconversion in zinc aluminate co-doped with Yb3+/Er3+ was achieved under continuous excitation at 980 nm at room temperature. The emission spectra of the samples indicated that red luminescence predominated over green in response to IR laser excitation. A comparison of the spectra of upconversion luminescence of all the co-doped compositions indicated that the 10:1 composition showed the lowest relative emission intensity due to self-removal of the ions involved, causing the distance between ions to decrease, which favored the occurrence of CR. The 5:1 composition showed better upconversion emission than the 2:1 and 10:1 compositions, probably due to the good accommodation of Yb3+/Er3+ ions in the zinc aluminate, favoring ET between the transition states. Zinc aluminate co-doped with a 5:1 concentration of Yb3+/Er3+ with intense upconversion fluorescence can be used as potential host material for upconversion lasers.
References
Feldmann C, Justel T, Ronda CR, Schmidt PJ (2003) Adv Funct Mater 13:511
Saines PJ, Elcombe MM, Kennedy BJ (2006) J Solid State Chem 179:613
Shanfeng L, Min Z, Yang P, Qingyu Z, Mingshan Z (2010) J Rare Earths 28(2):237
Zhou J, Zhang W, Huang T, Wang L, Li J, Liu W, Jiang B, Pan Y, Guo J (2011) Ceram Int 37:513
Yang Z, Zhu K, Song Z, Zhou D, Yin Z, Qiu J (2011) J Solid State Commun 151:364
Weng F, Chen D, Wang Y, Yu Y, Huang P, Lin H (2009) Ceram Int 35:2619
Qu Y, Kong X, Sun Y, Zeng Q, Zhang H (2009) J Alloy Compd 485:493
Ping H, Chen D, Yu Y, Wang Y (2010) J Alloy Compd 490:74
Chen L, Yu C, Hu L, Chen W (2012) Solid State Sci 14:e287
You W, Lai F, Jiang H, Liao J (2012) Physica B 407:1094
Chen XY, Ma C, Zhang ZJ, Wang BN (2008) Mater Sci Eng B 151:224
Menon S, Dhabekar B, Alagu Rajaa E, More SP, Gundu Raob TK, Kher RK (2008) J Lumin 128:1673
Chen XY, Ma C (2010) Opt Mater 32:415
Barros BS, Melo PS, Kiminami RHGA, Costa ACFM, Sá GF, Alves S Jr (2006) J Mater Sci 41:4744. doi:10.1007/s10853-006-0035-6
Shen SC, Hidajat K, Yu LE, Kawi S (2004) Adv Mater 16:541
Farhadi S, Panahandehjoo S (2010) Appl Catal A 382:293
Zawadzki M, Wrzyszcz J, Strek W, Hreniak D (2001) J Alloy Compd 323–324:279
Singh V, Chakradhar RPS, Rao JL, Kim D-K (2008) J Lumin 128(3):394
Jain SR, Adiga KC, Pai Verneker V (1981) Combust Flame 40:71
Costa ACFM, Kiminam RHGA, Morelli MR (2009) Handbook of nanoceramics and their based nanodevices, vol 5. American Science Publishers, Valencia, p 80
Klung H, Alexander L (1962) X-ray diffraction procedures. Wiley, New York
Sun H, Dai S, Xu S, Wen L, Hu L, Jiang ZJ (2004) Mater Lett 58:3948
Wang X, Kong X, Shan G, Yu Y, Sun Y, Feng L, Chao K, Lu S, Li Y (2004) J Phys Chem B 108(48):18408
Acknowledgements
The authors gratefully acknowledge the Brazilian Research Funding Agencies CNPq, RENAMI, FAPESP, and Inct-INAMI for their financial support of this work. We are also indebted to Prof. Luiz Antonio de Oliveira Nunes from the Department of Physics of the University of São Paulo-USP for performing the upconversion tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, A.C.F.M., Kiminami, R.H.G.A., Santos, P.T.A. et al. ZnAl2O4 co-doped with Yb3+/Er3+ prepared by combustion reaction: evaluation of photophysical properties. J Mater Sci 48, 172–177 (2013). https://doi.org/10.1007/s10853-012-6725-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6725-3