Abstract
A novel kind of incompletely condensed polyhedral oligomeric silsesquioxane (TAP-POSS) containing allyl groups was successfully synthesized, and its structure was characterized by fourier transform infrared, nuclear magnetic resonance (1H-NMR and 29Si-NMR) and X-ray diffraction. In addition, TAP-POSS was hybridized with 2,2′-diallylbisphenol A (DBA)-modified 4,4′-bismaleimidodiphenylmethane (BDM) resin to develop a new kind of hybrids with simultaneously improved thermal stability and dielectric properties. Compared with BDM/DBA resin, the BDM/DBA/TAP-POSS hybrids have obviously increased thermal resistance reflected by the increased glass transition temperature (T g) and char yield at high temperature. For example, the T g of modified BDM/DBA resin with only 3.0 wt% TAP-POSS is 330 °C, which is 36 °C higher than that of pure BDM/DBA resin. On the other hand, the dielectric properties over wide frequency and temperature ranges are thoroughly investigated, and results show that the dielectric constant and loss of all hybrids are not only lower than those of BDM/DBA resin over the whole frequency and temperature ranges tested, but also exhibit smaller dependence on temperature and frequency. These phenomena can be explained from the variety of cross-linked structure induced by the presence of TAP-POSS. The outstanding integrated properties of BDM/DBA/TAP-POSS hybrids suggest that TAP-POSS has advantages over conventional POSS, thus the method proposed herein is a new approach to develop high performance structural/functional materials for cutting-edge industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the last decade, the rapid development of microelectronic industry has brought increasing requirements on high performance insulating materials which are distinguished by good processing characteristics, higher thermal stability, better moisture resistance, and lower dielectric loss [1–3], and thus make it impossible for one type of material to meet these harsh requirements. Since last decade, inorganic/organic hybrids have attracted great attentions worldwide because they combine the advantages of both inorganic and organic phases [4–8], and show some new unique properties which are not possessed by either inorganic or organic phase.
Lots of investigations have proved that the properties of the hybrids are determined by the nature of inorganic and organic phases as well as the interface between the two phases, demonstrating that inorganic and organic phases should be carefully selected to meet the requirements of applications.
For selecting organic phase, high performance thermosetting resins show greater advantages than thermoplastics because the former has good processing characteristics and diversity of reactivity [9, 10]. In this study, bismaleimide (BMI) resin, one kind of representative heat resistant thermosetting resin, is chosen as the organic phase for developing hybrids owing to its outstanding properties and wide applications (for example, high performance adhesives and coatings, the matrices of advanced composites, etc.) in aerospace, microelectronic, and insulating fields [11, 12]. However, its thermal stability and dielectric properties still need to be further improved to fit the rapid development of modern microelectronic industry.
With regard to inorganic phase, polyhedral oligomeric silsesquioxane (POSS) and derivatives have attracted great attention owing to their unique nanometer-sized silica-like cage structure and properties as well as the possibility for functionalization with a wide variety of organic groups to adjust the interfacial adhesion with various polymers [13–16]. To date, many functional POSS derivatives with active groups such as hydrogen [17], amine [18], vinyl [19], and epoxy groups [20] have been synthesized to develop hybrids. However, the corresponding researches of the hybrids based on thermosetting resins mainly focus on phenolic and epoxy resins, few of which is related to BMI/POSS hybrids, probably because it is not easy to synthesize a desirable POSS with suitable functional groups which not only have good reaction with BMI, but also endow modified BMI resin with good performance. The representative work was carried out by Huang’s group, in which the hybrids consisting of 4,4′-bismaleimidodiphenolmethane (BDM), dipropargyl ether of bisphenol A (DPBPA), and octa(aminophenyl)silsesquioxane (OAPS) were prepared, and the effect of the content of OAPS on the thermal properties of the hybrids was studied. Results show that compared with BDM/DPBPA resin, the hybrid with 5 wt% OAPS has significantly higher glass transition temperature but decreased thermal stability owing to the instability of N–C bond under high temperature [21].
Incompletely condensed POSS (IC-POSS) is one kind of POSS derivative which inherits many advantages of complete condensed POSS including excellent thermal resistance, lower dielectric constant and loss, etc., but also displays unique structure. As we’ve known that, generally, a completely condensed POSS is terminated with same functional groups, for example, octavinylsilsesquioxanes, decaphenylsilsesquioxanes, etc., hence it is not easy to give consideration to multi-performance. However, this problem can be overcame by synthesizing IC-POSS, that is, a IC-POSS molecule can be terminated by two different functional groups [22], and thus shows a unique advantage for developing novel materials.
To date, some IC-POSS derivatives have been synthesized and used to prepare hybrids with polybenzoxazine [23], polycarbonate [24], polyimide [25], phenolic resin [26], or cyanate ester [27], etc. Results show that these hybrids have greatly improved thermal and mechanical properties. However, the active groups of these IC-POSS derivatives are silanol groups, which are not suitable to prepare hybrids with BMI resin. On the other hand, favorable dielectric properties are known to be the key properties of high performance insulating materials [28], so it is necessary to investigate the dielectric properties when developing new high performance hybrids, but no corresponding work has been done on these hybrids based on IC-POSS. In detail, many dielectric materials (for example computer circuits, radars) need to operate at different frequencies at different locations, hence these dielectric materials with stable dielectric constant and loss across a large frequency range are preferred. In addition, outstanding thermal resistance is an index of heat resistant resins, and hence it is necessary to study the influence of temperature on the dielectric property. However, unfortunately, no literature reported this subject; therefore, it is of great interests to synthesize new IC-POSS with suitable functional groups for developing novel BMI resin-based hybrids, and systematically investigate the integrated performance, especially dielectric properties.
During last three decades, lots of researches have been conducted on the modifications of BMI resin, and results show that co-polymerizing with allylphenyl compounds is an effective technique for developing high performance BMI resins [29]. In this article, a new incompletely condensed POSS containing allyl groups (TAP-POSS) is designed and synthesized, moreover, which is used to prepare novel hybrids with BMI resin. In addition, the structure and property of the hybrids are systematically evaluated for building up a new approach for developing high performance hybrids.
Experimental
Materials
BDM was obtained from Institute of Northwestern Chemical Engineering (China). 2,2′-Diallylbisphenol (DBA) was purchased from Laiyu Chemical Factory (China). Phenyltrimethoxysilane was supplied by Zhejiang Chem-tech Group Co. Ltd (China). Sodium hydroxide with analytic purity was purchased from Shanghai Reagent Co. (China). Tetrahydrofuran (THF) and acetone were obtained from Shanghai Reagent Co. (China), which were dried before use. Other reagents were commercial products with analytic grades, and used as received.
Heptaphenyltricycloheptasiloxane trisodium silanolate (HPSTS) was prepared according to the method described in literature [30, 31]. FTIR (cm−1, KBr): 3044, 1589, 1431, and 1116–1050 (Si–Ph); 1050–1000 (Si–O–Si). 1H NMR (ppm): 7.31–7.80 (Ph–H). 29Si NMR (ppm): −77.71 (O3SiPh); −76.53 (O2SiPhNa).
Synthesis of TAP-POSS
TAP-POSS was synthesized following a two-step mechanism as shown in Scheme 1. HPSTS (2 g, 2 mmol) was dissolved in 100 mL anhydrous acetone to form a solution, and then allyl bromide (1.45 g, 12 mmol) was quickly added to the solution with vigorous stirring. The nucleophilic substitution reaction was carried out following a procedure of 30 °C/3 h + 50 °C/3 h + 60 °C/3 h. When the reaction was over, the white solid (NaBr) was removed via vacuum filtration to get the residual solution. After that solvents were distilled in vacuum to leave a pale yellow solid which was washed with distilled water to obtain a crude product. The crude product was dried in vacuo at 50 °C for 48 h to obtain the target product (TAP-POSS), of which the molecular formula is (CH2CHCH2)3O12Si7(C6H5)7.
FTIR (cm−1, KBr): 3044, 1589, 1431, and 1116–1050 (Si–Ph); 1050–1000 (Si–O–Si); 2920, 2848, and 1648 (–CH2–CH=CH2).
1H NMR (ppm): 7.30–7.82 (Ph–H); 4.46, 5.09, and 5.86 (–CH2–CH=CH2).
29Si NMR (ppm): −78.89 (O3SiPh); −77.84 (O2SiPhCH2CHCH2).
Preparation of BDM/DBA prepolymer
According to Table 1, appropriate quantities of DBA and BDM were added into a beaker with a mechanical stirrer. The mixture was heated to 125–130 °C and maintained within that temperature range with stirring until a clear and brown liquid was obtained. The liquid was maintained at that temperature for additional 15 min to obtain a transparent liquid, which was BDM/DBA prepolymer.
Preparation of BDM/DBA/TAP-POSS prepolymer
According to Table 1, appropriate quantities of DBA and TAP-POSS were added into a beaker, the mixture was heated to 125–130 °C and maintained within that temperature range with stirring until TAP-POSS was completely dissolved. And then appropriate quantity of BDM was added into the mixture slowly, and kept at that temperature with stirring until a clear and brown liquid was obtained. The liquid was maintained at that temperature for additional 15 min to obtain a transparent liquid, which was BDM/DBA/nTAP-POSS prepolymer, where n refers to the content of TAP-POSS in the hybrid.
Preparation of cured BDM/DBA resin and BDM/DBA/TAP-POSS hybrids
Each prepolymer was completely degassed under vacuum at 120 °C and poured into a preheated (120 °C) glass mold, and then the prepolymer was cured and postcured following the procedures of 130 °C/2 h + 150 °C/2 h + 180 °C/2 h + 200 °C/2 h + 220 °C/2 h, and 230 °C/4 h, successively.
Measurements
Fourier Transform Infrared (FTIR) spectra were recorded between 400 and 3500 cm−1 with a resolution of 2 cm−1 on a Nicolet FTIR 5700 spectrometer (USA). The sample was pressed into a pellet with KBr.
1H nuclear magnetic resonance (1H NMR) spectra were obtained using an NMR system (300-MHz NMR spectrometer, USA) with acetone-d6 as the solvent, and tetramethylsilane as internal standard.
29Si NMR spectra were recorded on a Bruker WM300 (Germany) using the tetramethylsilane (CH3)4Si as the standard substance. The spectrum of Na3O12Si7(C6H5)7 was recorded with acetone-d6 as the solvent, and that of TAP-POSS was recorded with methylbenzene-d8 as the solvent.
X-ray diffraction (XRD) analyses were carried out on a MERCURY CCD X-ray diffractometer (RIGAKu, Japan) with CuKα radiation. The 2θ angle ranged from 5° to 70°, and the scan rate was 2°/min.
Differential scanning calorimeter (DSC) measurements were performed using a DSC 2010 (TA Instruments, USA) at a heating rate of 10 °C/min under a nitrogen atmosphere.
The dielectric properties were measured using a Broadband Dielectric Spectrometer (Novocontrol Concept 80 analyzer, Germany). The dimensions of each sample were (25 ± 0.02) × (25 ± 0.02) × (3 ± 0.01) mm3.
Thermogravimetric (TG) analyses were performed using PerkinElmer TGA-7 (USA) at a heating rate of 10 °C/min in a nitrogen atmosphere from 50 to 800 °C. The initial decompose temperature (T di) was defined as the point of intersection at which the tangent of onset temperature and the tangent of the maximum degradation rate temperature.
Dynamic mechanical analysis (DMA) scans were performed in single-cantilever blending mode using a TA Instruments dynamic mechanical analyzer (DMA Q800, USA) from 50 to 350 °C with a heating rate of 3 °C/min at 1 Hz.
Results and discussion
Structure of TAP-POSS
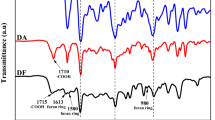
Figure 1 shows the FTIR spectra of HPSTS and TAP-POSS. Compared with the spectrum of HPSTS, there are three new peaks in the spectrum of TAP-POSS at 2920, 2848, and 1648 cm−1, assigning to the stretching vibration of C–H, –CH2– and C=C groups, respectively, suggesting the successful grafting of allyl groups in the molecule of TAP-POSS. This statement can be further confirmed by the 1H-NMR spectra of TAP-POSS and HPSTS as shown in Fig. 2. It can be seen that both spectra show the broad chemical shift from 7.1 to 7.82 ppm attributing to the silica-like cage, indicating that the cage structure is not changed during the synthesizing procedure. However, compared with the spectrum of HPSTS, the 1H NMR spectrum of TAP-POSS has three chemical shifts at 4.46, 5.09, and 5.86 ppm, ascribing to the protons of –CH2–, =CH2, and –CH=, respectively, suggesting that TAP-POSS has allyl groups (Scheme 2).
29Si NMR is a useful technique for characterizing the structure of silicone. Figure 3 shows the 29Si NMR spectra of TAP-POSS and HPSTS, each spectrum shows two peaks, indicating that silicon atoms of TAP-POSS and HPSTS have two structural conformations, or incompletely closed POSS structure [32]. Note that the two 29Si NMR spectra have no significant difference, suggesting that the cage arrangement of silsesquioxane does not occur under this reaction condition. Based on these results, it is reasonable to state that the target product (TAP-POSS) was successfully obtained.
Figure 4 shows the XRD patterns of HPSTS and TAP-POSS. Both curves exhibit two diffraction peaks. In detail, the sharp crystalline peak appearing at about 2θ = 7.0° is attributed to the cage-like structure, and the broad diffraction peak at about 2θ = 18.7° is assigned to the amorphous structure, implying that HPSTS and TAP-POSS are incompletely cage-like structure. In addition, the diffraction peak of TAP-POSS appears at slightly higher angles, suggesting that the crystal structure of TAP-POSS is not completely similar as that of HPSTS.
The curing behavior and cured structure of BDM/DBA/TAP-POSS hybrids
For a thermosetting system, its curing behavior determines the structure, and thus the performance of cured resin, so it is necessary to first investigate the effect of TAP-POSS on the curing behavior and cured structure of BDM/DBA resin.
In order to evaluate the reactivity of TAP-POSS on the whole curing reaction of BDM/DBA, the DSC curves of BDM/DBA and BDM/DBA/TAP-POSS prepolymers were measured, and shown in Fig. 5. Each prepolymer exhibits one exothermic peak at about 250 °C, indicating that BDM/DBA/TAP-POSS prepolymer has the same curing mechanism as BDM/DBA prepolymer. Specifically, besides the self-polymerization of BDM via C=C bonds [29, 33], there are ‘Ene’ and ‘Diels–Alder’ reactions between maleimide and allyl groups, which provides a chemical bonding between TAP-POSS and BDM/DBA to guarantee a good dispersion of TAP-POSS in the matrix as well as the good interfacial adhesion between BDM/DBA resin and TAP-POSS.
Besides the chemical structure, the cross-linking density (ρ) is another important parameter characterizing the cured structure of a thermosetting resin. In order to confirm the effect of TAP-POSS on the ρ, the ρ values of BDM/DBA resin and BDM/DBA/TAP-POSS hybrids were calculated by the classical equation based on the statistical theory of rubber elasticity [34] as shown in Eq. 1:
where G′ is the storage modulus (Fig. 6) of the sample at the temperature T from DMA analyses; Φ is the front factor, and assumed to be 1; T is the absolute temperature at which the sample is in rubbery state, herein T is selected as the temperature which is 20 °C higher than the glass transition temperature (T g); and R is the gas constant.
The corresponding ρ values of BDM/DBA resin and hybrids are summarized in Fig. 7. It can be seen that all hybrids have bigger ρ values than BDM/DBA resin; moreover, the ρ value of the hybrid increases as the content of TAP-POSS increases. As discussed above that BDM/DBA/TAP-POSS hybrids have similar curing mechanism as BDM/DBA resin; moreover, the mole ratios between imide and allyl groups for hybrids and BDM/DBA resin are equal (Table 1); therefore, the difference in molecular structure between DBA and TAP-POSS plays a main role in the ρ value of each cross-linked network. Three allyl groups exist on a nano cage structure of one TAP-POSS molecule, while two allyl groups exist in a relatively “long” molecular chain of DBA, consequently, the presence of TAP-POSS tends to shorten the distance between the cross-linked points, and thus increases the ρ value of the resultant network.
Thermal resistance of cured BDM/DBA/TAP-POSS hybrids
BMI resin is famous for its outstanding thermal resistance [35]; therefore, the merit should be maintained when developing new materials based on BMI resin. Thermal resistance is generally evaluated by the thermal degradation behavior and T g, reflecting the thermal stability of molecular chains, and the movement capacity of molecular chains with the increase of temperature, respectively.
DMA technique is a very effective method to detect the T g value of a thermosetting resin [36]. The peak temperature of loss modulus–temperature plot is defined as the T g herein. Figure 8 shows the overlay plots of loss modulus versus temperature for cured BDM/DBA resin and BDM/DBA/TAP-POSS hybrids, it is noted that T g value increases with increasing the content of TAP-POSS. For example, the whole peak of BDM/DBA/3%TAP-POSS shifts toward higher temperature by about 36 °C. Similar results have been found for other hybrids based on POSS, but their increments are generally not bigger than 30 °C [23, 27]. The outstanding increment in T g value for BDM/DBA/TAP-POSS hybrids is proposed to be contributed to the change in the structure of cross-linked network induced by the addition of TAP-POSS. First, the nanocage of TAP-POSS is not only relatively rigid, but also has co-reaction with BDM/DBA resin, and thus tending to greatly restrict the segment’s motion of BDM/DBA resin. Second, compared with the resin, the hybrids have higher cross-linking densities, which is also beneficial to improve the T g value.
Figure 9 gives the TG and DTG curves of cured BDM/DBA resin and BDM/DBA/TAP-POSS hybrids. The typical data, such as the initial decompose temperature (T di), the temperature of the maximum degradation rate (T max), and char yield (Y c) at 800 °C, obtained from these curves are summarized in Table 2. BDM/DBA/TAP-POSS hybrids show similar shapes of TG and DTG curves, suggesting that they have similar degradation mechanism. On the other hand, as discussed above that cured BDM/DBA resin and BDM/DBA/TAP-POSS hybrids have somewhat different chemical structures, however, they have same segments with the poorest thermal stability owing to the similar curing mechanism, therefore, it is reasonable to observe that the hybrids exhibit similar T di and T max values as the resin, because the thermal degradation is mainly dependent on the segments with the poorest thermal stability of the cross-linked network.
All BDM/DBA/TAP-POSS hybrids have obvious higher Y c values at 800 °C than BDM/DBA resin, and the larger is the content of TAP-POSS, the higher is the Y c value. This increase is mainly attributed to the high Y c value of POSS. Liu’s group found that during the process of thermal degradation for POSS-based resin, the organic substituent on POSS first undergoes the hemolytic Si–C bond cleavage, and then is the fusion of POSS to form a thermally insulated and oxidatively stable silica layer [37]. When the content of TAP-POSS is small, a continuous silica protective barrier can not be formed on the surface of the sample. That is to say, a larger content of TAP-POSS tends to form a continuous silica protective barrier, leading to higher Y c value and thermal stability. That is also the reason why BDM/DBA/3.0%TAP-POSS hybrid has an significantly increased Y c value.
On the other hand, it is noteworthy that all experimental Y c values of hybrids are bigger than their respective theoretical values calculated by the “Mixture Rule”, and this phenomenon is enhanced as the content of TAP-POSS increases, demonstrating that there is a synergistic effect in the hybrids which is believed to come from the interaction between TAP-POSS and BDM/DBA resin.
Dielectric property of BDM/DBA/TAP-POSS hybrids
High performance insulating materials for micro-electric applications are preferred to have excellent dielectric properties including low and stable dielectric constant and loss values across large frequency and temperature ranges, and thus achieve desirable usage reliability of products [38]. Therefore, it is interesting to systematically investigate the temperature and frequency dependence of dielectric properties for BDM/DBA/TAP-POSS hybrids.
Dependence of dielectric constant on frequency and temperature
Figure 10 shows three-dimensional plots of dielectric constant–temperature–frequency of cured BDM/DBA resin and BDM/DBA/TAP-POSS hybrids. Over the whole temperature and frequency ranges tested herein, all hybrids have lower dielectric constants than BDM/DBA resin, and that with a larger content of TAP-POSS has lower dielectric constant. It can be concluded from above discussion that the addition of TAP-POSS to BDM/DBA resin brings complex influences on the structure of cross-linked network, that is, the increased cross-linking density, the porous structure of TAP-POSS and higher T g values are believed to play the decisive role for the decreased dielectric constant of hybrids.
Dielectric constant of a polymer is determined by the orientation and relaxation of dipoles of the polymer [39], which is closely related to the motion of molecular chain segments. As the motion of polymer chain segments is greatly dependent on the temperature, hence the dielectric constant of a polymer is significantly influenced by the temperature. According to this concept, it is reasonable to observe that with the increase of the temperature, the dielectric constant of either cured BDM/DBA resin or BDM/DBA/TAP-POSS hybrid increases, but the increment degree of hybrids is greatly dependent on the temperature and the content of TAP-POSS. Specifically, when the temperature is not higher than 150 °C, the dielectric constants of cured BDM/DBA resin and BDM/DBA/TAP-POSS hybrids are very stable over the whole frequency range, and only increase slightly as the temperature increases. For example, when the temperature increases from −50 to 150 °C, the dielectric constant at 1 Hz of cured BDM/DBA resin and that of BDM/DBA/3.0%TAP-POSS hybrid enlarges about 4.8 and 5.8%, respectively. However, when the temperature is 200 °C or higher, as the temperature increases, the dielectric constant at low frequency (<10 Hz) of each sample (especially cured BDM/DBA resin) not only obviously increases, but also exhibits greater dependence on the frequency, that is, the dielectric constants (1 Hz) at 250 °C for cured BDM/DBA resin and BDM/DBA/3.0%TAP-POSS hybrid are 5.94 and 4.39, about 1.6 and 1.2 times of that at 50 °C, respectively. This is because that at high temperature (around T g), the polymer chain segments become much active and mobile, and thus the dipoles are more easily aligned in response of the applied electric field, leading to higher dielectric constant. Note that dipoles in the viscoelastic polymers can get longer time to align at low frequency, meaning that more polarizations of dipole orientation contribute to the dielectric constant, and thus leading to the phenomenon that dielectric constant has stronger sensitivity to frequency at low frequency, and is almost independent on frequency at high frequency. Compared with BDM/DBA resin, BDM/DBA/TAP-POSS hybrids have higher T g values, so the dielectric constant of BDM/DBA resin is bigger than that of hybrids at the same temperature, and the latter shows less frequency dependence. In other words, a larger content of TAP-POSS is beneficial to get lower and more stable dielectric constant over the wide frequency (1–106 Hz) and temperature (from −50 to 300 °C) range.
Dependence of dielectric loss on frequency and temperature
Figure 11 shows three-dimensional plots of dielectric loss–temperature–frequency of cured BDM/DBA resin and BDM/DBA/TAP-POSS hybrids. It can be seen that dielectric loss shows similar dependence on temperature and frequency as dielectric constant because the parameters affecting the dielectric constant usually also play role on dielectric loss [40]. However, dielectric loss also exhibits some differences with dielectric constant. First, the temperature for the appearance of significantly increased dielectric loss is 150 °C, which is lower than that for dielectric constant, indicating that dielectric loss is more temperature-dependent. In addition, the increment degree of dielectric loss is much bigger than that of dielectric constant. For example, the dielectric loss (1 Hz) at 250 °C for cured BDM/DBA/3.0%TAP-POSS hybrid and BDM/DBA resin are about 80 and 135 times of that at 50 °C, respectively. These phenomena can be ascribed to their physical meanings. Specifically, dielectric constant measures the polarization of the medium per unit in an applied electric field; while dielectric loss represents the energy dissipated per cycle in an applied field, associating with the energy loss during the time-dependent polarization, hence both of them are closely related to the amount, strength, and movement ability of polar groups. However, dielectric constant represents the whole orientation and relaxation of dipoles, meaning that the orientation and relaxation of dipoles in a symmetry structure will counteract each other, and thus resulting in low dielectric constant; however, any orientation and relaxation of dipoles, even in a symmetry structure, will combine together, leading to big dielectric loss.
Second, it is interesting to observe that there is a upward peak in the curve of dielectric loss–frequency of each sample at −50 °C, the peak shifts toward high frequency as the temperature increases. For example, when the temperature increases from −50 to 0 °C (or 50), the maximum dielectric loss of cured BDM/DBA resin shifts from 103 to 105 (or 106) Hz. This phenomenon can be explained from the influence of temperature on the molecular motion, and the dependence of frequency on orientation and relaxation of dipoles. In detail, at very low temperature, the polymer molecule is in “frozen” state, so its motion ability is very low, meaning that the molecule only follows up the step of the applied electric field in a low frequency range. As a result, when the frequency increases from very low value, the dielectric loss initial increases owing to the occurrence of the orientation of dipoles, but which will quickly reach the maximum value because the molecule can not follow up the applied electric field even when the increased frequency is still not high, and consequently dielectric loss decreases. As the temperature increases, the ability of the molecular movement enhances, hence the frequency at which the molecule can follow up the applied electric field enlarges, and the energy loss at the same frequency during the orientation tends to decrease, as a result, the maximum peak of dielectric loss shifts toward high frequency.
Conclusions
A new kind of incompletely condensed TAP-POSS containing allyl groups is synthesized, and then a new kind of high performance BDM/DBA/TAP-POSS hybrids with significantly improved thermal resistance and dielectric properties is developed. BDM/DBA/TAP-POSS prepolymer has the same curing mechanism as BDM/DBA prepolymer, but the former has bigger ρ value than the latter, and the ρ value of the hybrid increases as the content of TAP-POSS increases. These differences in the cross-linked network are responsible to the significantly improved thermal and dielectric properties of BDM/DBA/TAP-POSS hybrids. The outstanding integrated performance of BDM/DBA/TAP-POSS hybrids shows a great potentiality to be used as high performance electronic composites for applications needing harsh requirements in thermal and dielectric properties.
References
Hu JT, Gu AJ, Liang GZ, Zhuo DX, Yuan L (2011) Express Polym Lett 5:558
Ahner N, Schulz SE, Blaschta F, Rennau M (2007) Microelectron Eng 84:2606
Karad SK, Attwood D, Jones FR (2005) Composites 36:764
Pankow O, Gudrun SN (2004) Macromol Mater Eng 289:990
Mamunya YP, Iurzhenko MV, Lebedev EV, Ischenko SS, Boiteux G, Seytre G (2007) J Non-Cryst Solids 353:4288
Ren PG, Liang GZ, Zhang ZP, Lu T (2006) Composites 37:46
Li QF, Xu YH, Yoon JS, Chen GX (2011) J Mater Sci 46:2324. doi:10.1007/s10853-010-5077-0
Lim SK, Hong EP, Song YH, Choi HJ, Chin IJ (2010) J Mater Sci 45:5984. doi:10.1007/s10853-010-4446-z
Zhang BY, Li M, Chen XB (2007) J Mater Sci 42:9170. doi:10.1007/s10853-007-1920-3
Wooster TJ, Abrol S, Hey JM, MacFarlane DR (2004) Composites 35:75
Rajasekaran R, Alagar M, Karikal CC (2008) Express Polym Lett 2:339
Fang ZP, Shi HH, Gu AJ, Feng Y (2007) J Mater Sci 42:4603. doi:10.1007/s10853-006-0543-4
Seino M, Hayakawa T, Ishida Y, Kakimoto M (2006) Macromolecules 39:3473
Nagendiran S, Alagar M, Hamerton I (2010) Acta Mater 58:3345
Huang JM, Kuo SW, Huang HJ, Wang YX, Chen YT (2009) J Appl Polym Sci 111:628
Monticelli O, Waghmare P, Chincarini A (2009) J Mater Sci 44:1764. doi:10.1007/s10853-009-3281-6
Crivello JV, Mallk R (1997) J Polym Sci 35:407
Tamaki R, Choi J, Laine RM (2003) Chem Mater 15:793
Zhang CX, Babonneau F, Bouhomme C, Laine RM, Soles CL, Hristov HA, Yee AF (1998) J Am Chem Soc 120:8380
Wang YZ, Tsai HS, Ji ZY, Chen WY (2007) J Mater Sci 42:7611. doi:10.1007/s10853-007-1845-x
Huang FW, Rong ZX, Shen XN, Huang FR, Du L, Li ZP (2008) Polym Eng Sci 48:1022
Deng JJ, Polidan JT, Hottle JR, Farmer-Creely CE, Viers BD, Esker AR (2002) J Am Chem Soc 124:15194
Du WJ, Shan JJ, Wu YX, Xu RW, Yu DS (2010) Mater Des 31:1720
He QL, Song L, Hu Y, Zhou S (2009) J Mater Sci 44:1308. doi:10.1007/s10853-009-3266-5
Verker R, Grossman E, Gouzman I, Eliaz N (2009) Compos Sci Technol 69:2178
Zhang YD, Lee S, Yoonessi M, Liang KW, Pittman CU (2006) Polymer 47:2984
Liang KW, Li GZ, Toghiani H, Koo JH, Pittman CU Jr (2006) Chem Mater 18:301
Gu AJ (2006) Compos Sci Technol 66:1749
Devi KA, Nair CPR, Ninan KN (2007) J Appl Polym Sci 106:1192
Ni Y, Zheng SX (2007) J Polym Sci 45:1247
Koh K, Sugiyama S, Morinaga T, Ohno K, Tsujii Y, Fukuda T, Yamahiro M, Iijima T, Oikawa H, Watanabe K, Miyashita T (2005) Macromolecules 38:1264
DW LEE, Kawakami Y (2007) Polym J 39:230
Rozenberg BA, Dzhavadyan EA, Morgan R, Shin E (2002) Polym Adv Technol 13:837
Zhang BF, Wang ZG, Zhang X (2009) Polymer 50:817
Anuradha G, Sarojadevi M, Sundararajan PR (2008) J Appl Polym Sci 110:517
Bussu G, Lazzeri A (2006) J Mater Sci 41:6072. doi:10.1007/s10853-006-0694-3
Liu YR, Huang YD, Liu L (2006) Polym Degrad Stab 91:2731
Duan JK, Kim C, Yun Z, Jiang PK (2008) J Appl Polym Sci 110:3096
Fan J, Hu X, Yue CY (2003) J Polym Sci 41:1123
Huang PZ, Gu AJ, Liang GZ, Yuan L (2011) J Appl Polym Sci 121:2113
Acknowledgements
The authors thank the National Natural Science Foundation of China (50873073, 20974076), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Major Program of Natural Science Fundamental Research Project of Jiangsu Colleges and Universities (11KJA43001), ‘‘Qing Lan Project’’ (2008) of Jiangsu Province in China, and Suzhou Applied Basic Research Program (SYG201141) for financially supporting this project.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zeng, L., Liang, G., Gu, A. et al. High performance hybrids based on a novel incompletely condensed polyhedral oligomeric silsesquioxane and bismaleimide resin with improved thermal and dielectric properties. J Mater Sci 47, 2548–2558 (2012). https://doi.org/10.1007/s10853-011-6078-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-6078-3