Abstract
For the ternary (60 − x)V2O5–40TeO2–xNiO glasses with 0 ≤ x ≤ 20 (in mol%), the glass transition temperature (T g) and crystallization temperature (T cr) have been determined using differential scanning calorimetry (DSC) at heating rates φ = 3, 6, 9, and 12 K/min. The effect of the heating rate and the NiO content on T g is discussed. It was observed that the transition region shifts to higher temperatures when the measuring time is reduced (or, conversely, when the applied temperature rate is increased). Using DSC, the compositional dependence of T g has been determined and so, an empirical equation has been deduced which relates the glass transition temperature with the NiO content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Physical and structural properties of multicomponent tellurite glasses were mainly studied due to their practical applications and also some interesting features such as their low melting temperature, no hygroscopic property, and good infra-red transmission [1–18]. Study in structural features of glasses by DSC investigation is a suitable way to understand the behavior of glasses as a function of composition [19]. Investigation of the role of changing in glass composition on the thermal properties, the concentration of non-bridging oxygen, rigidity, and packing of the structure of glass can help us to reach to optimized composition satisfying high thermal stability against thermal shocks for technological applications. The nature of glass transition is a fundamental issue in the condensed matter physics [20–25]. In spite of extensive research devoted to understand the phenomenon of glass transition, there is no satisfactory description of this phenomenon and more study is needed to overcome this difficulty. DSC technique is widely used to investigate the glass transformation in glassy materials. The kinetics of the glass transition, as studied by the DSC method, is important in investigating the nature of the glass transformation process. The glass transition temperature can be accurately determined by DSC measurements. Moreover, the kinetic aspect of the glass transition is evident from the strong dependence of T g on the heating rate [20–24]. It can be observed that the transition region shifts to higher temperatures if the measuring time is reduced (or, conversely, if the applied temperature rate is increased) [26, 27].
To the best of our knowledge, there are some articles on the calorimetric properties of TeO2-based glasses and then, tricomponent glass systems of the form AmOn–TeO2–V2O5 (AmOn is an another oxide) have been studied [14–18, 28]; in this study, due to the importance of the binary TeO2–V2O5 glass, the calorimetric properties of V2O5–NiO–TeO2 glasses are studied. Thus, this article reports the measurements of glass transition temperature behavior for several V2O5–NiO–TeO2 glasses. In my previous article [8], the optical and electrical and some structural properties of the present samples have been investigated, but there is not any report on their glass transition nature. In summary, the objectives of this work are: (1) to investigate the effect of heating rate on the glass transition temperature of the amorphous V2O5–NiO–TeO2 samples and (2) to investigate the compositional dependence of the glass transition temperature.
Experimental procedure
Reagent grade (99.99%) chemicals V2O5, TeO2, and NiO were well mixed in prescribed composition in a mortar. Each glass batch (16 g) was melted in air in an alumina crucible in an electric furnace (ATBIN ALF15). Therefore, the ternary (60 − x)V2O5–xNiO–40TeO2 glasses with 0 ≤ x ≤ 20 (in mol%), hereafter termed as 40TVNx, were prepared by standard melt quenching technique. This compositional range was chosen primarily for the reason that the melts for these compositions are relatively easy to form glass. The melts for the compositions containing either x > 20 crystallize during quenching.
During the sample production, the melt was mixed every 5 min to prevent the separation of the three components. The melt was poured on to a polished steel block and immediately pressed by another, where the blocks were kept at room temperature. All of the obtained bulk samples were annealed to eliminate the mechanical stresses resulting from the quenching [29, 30]. The characterization of the glass systems was carried out by X-ray diffraction (XRD) studies using a Bruker diffractometer (AXS D8 Advance, Cu Kα, Germany). The density (ρ) of each sample was calculated by the Archimedes’s method using para-xylene as immersion liquid; results of density, XRD patterns have been previously reported [7]. Also, the glass transition temperature of these samples was obtained using DSC (Pyris1, USA). For each sample, the heating rates (φ) of 3, 6, 9, and 12 K/min were used to record the DSC curves.
Results and discussion
XRD patterns
The XRD characterization of 40TVNx samples has been carried out on different samples, confirming the amorphous nature of them; results have been reported in our previous study [8].
Thermal analysis
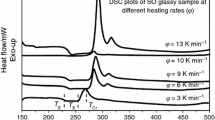
The representative DSC outputs of the 40TVN0 at different heating rates are shown in Fig. 1. The obtained data for other samples are listed in Table 1. In the absence of thermal events, the position of the baseline in such a plot is proportional to the specific heat of the sample. The presence of an endothermic peak, superimposed on the baseline, indicates the occurrence of a heat-absorbing event such as glass transition or melting. On the other hand, an exothermic peak occurs as a result of some sort of heat-releasing event such as crystallization [31]. The glass transition temperature as defined by the endothermic change in the DSC trace indicates a large change of viscosity, marking a transformation from amorphous solid phase to supercooled liquid state.
As the output of the DSC during heating is proportional to the heat capacity, it is a straightforward and convenient method of detecting the glass transition and investigating of its kinetics [26, 32–35]. For example, the heating-rate dependence of T g can be investigated. In this study, the middle point of the endothermic trace was used to define T g. Other definitions for T g were used by different authors. For instance, Abu-Sehly et al. [33] and Moynihan et al. [26] used different definitions of T g that included the extrapolated onset, the inflection point and the maximum point of the endothermic trace. The exothermic peak temperature T cr is used to identify the crystallization process. Both T cr and T g shift to higher temperatures with increasing heating rate. The heating-rate dependence of T g is clearly seen in Fig. 2, which indicates its increasing with increasing of φ. The obtained DSC values of T g at different heating rates for the other compositions show similar behavior.
The kinetic aspect of the glass transition is evident from the pronounced shift in T g. It is worth observing that an order of magnitude increase in φ causes a shift in T g of ~5 K [36].
The general features of DSC plots for the 40TVN10 glass indicate a wide range between the glass transition and the crystallization temperatures (see Fig. 1).
The curves in Fig. 2 indicate somewhat a nonlinear variation of T g against rate of heating. On the other hand, the DSC data reported in Table 1 show that for the different compositions, the glass transition temperature increase with increasing nickel oxide content in all heating rates. This variation is shown in Fig. 3, where T g is seen to increase with increasing x or with decreasing V content at each heating rate. Furthermore, using the results presented in Fig. 3, T g data show that the glass transition temperature is sensitive to the NiO concentration. Increasing of T g can be interpreted as increasing of the thermal stability of the glass. The thermal stability of the glass is a result of the glass structure; in other word, in this study, the change in T g indicates a change related to the manner in which V2O5 and NiO get arranged in the glass. The thermally stable glasses will have close packed structure, while the unstable glasses will have loose packed structure [37]. Thus, the addition of NiO increases the stability of the glass and the rigidity of the network, which is in agreement with the data of the glass density (a criterion of packing) (reported in [8]); glasses with higher thermal stability has probably good resistance against thermal shocks and so are suitable candidate for technological applications. It has been reported that in binary tellurite glass systems of the forms (100 − x) TeO2–xV2O5 (with x = 10, 20, 25, 30, 35, 40, 45, and 50 mol%), samples with higher percentage of V2O5 have the lower values of T g, i.e., V2O5 creates a more weaken tellurite glass [38]; as explained before, the present ternary glasses, similar behavior is seen and T g decreased with increasing of V2O5 content (i.e., substitution of V2O5 with NiO led to increasing of T g and then more thermal stability). One can compare the T g data of the present ternary samples with previously reported data for binary (100 − x) TeO2–xV2O5 glasses [38], which are in agreement for near compositions.
It has been reported [8] that the data of density ρ increase with increase in NiO content. Since nickel oxide has a high relative molecular mass, thus, it is an expected result.
This means that the glass transition temperature increases if the average coordination number increases. This may be due to the decrease in the number of V–V bonds and the increase of the V–Ni bonds as a result of the increasing of the NiO content (x) and the decrease of the V content. In other word, the cross-linking provided by Ni atoms increases for 40TVNx samples, which in turn affects the structure in a manner to increase the T g. By plotting ln(T g) versus x 3/2, we find straight lines with different slopes, as shown in Fig. 4. It is found that the slope A of the straight lines depends on the heating rate φ, which it has a positive value and that it increases with increasing φ. This means that the rate of variation of T g with x is larger for the lower heating rate. The dependence of A on the heating rate φ is shown in Fig. 6. It can be concluded that the relation between T g and x within the investigated ranges obeys an empirical equation of the Arrhenius form as follows:
where A and B are constants depending on φ. The values of A and B have been obtained from the slope and extrapolation of the linear relation in Fig. 4 to x 3/2 = 0, respectively (see also Table 1). The values of A and B can be derived at any desired heating rate within the measured range from Figs. 5 and 6, respectively; in Figs. 5 and 6, the variation of A and B can be estimated by using the equations A = (α′ ln(φ−3/4) + β′ and B = α lnφ + β, respectively (as shown in the inset of these figures).
Generally, it is emphasized [26, 39] on the role of shear modulus as an important thermodynamic and kinetic parameter governing the properties and relaxation of the glassy state; furthermore temperature dependence of shear modulus do not depend on heating rate at T > T g; in other word, at T < T g shear viscosity depends on heating rate and is responsible for structural relaxation and related viscoelasticity [39]. Thus, it seems that the thermal stability and so T g is related to the shear viscosity [26]; now, there is experimental or theoretical data available for shear modulus to be studied. Heating rate dependence of T g is somewhat an open question also for simple systems and even more for the complex system. On the other hand, the considerable theoretical difficulties experienced for amorphous solids are amplified by the lake of adequate experimental information; thus authors attempt in search of more insight into thermal stability, calorimetric behavior, and fundamental explanations to full this gap.
Finally, one can conclude and determine the effect of heating rate on the glass transition temperature.
Conclusions
Investigation of heating-rate dependence of the glass transition temperature in (60 − x)V2O5–xNiO–40TeO2 glasses was carried out using DSC technique. The increase of glass transition temperature with NiO content in the investigated system obeys an equation of the Arrhenius form. The increase of T g with increasing nickel oxide content may be due to an increase of the number of Ni–V bonds.
T g depends on heating rate and show an increasing trend. The compositional dependence of T g has been identified for the present samples.
References
El-Mallawany R (2002) Tellurite glass handbook: physical properties and data. CRC Press, FL
Stanworth JE (1952) J Soc Glass Technol 36:217
Souri D (2010) J Non-Cryst Solids 356:2181
Souri D, Shomalian K (2009) J Non-Cryst Solids 355:1597
Souri D, Salehizadeh SA (2009) J Mater Sci 44:5800. doi:https://doi.org/10.1007/s10853-009-3814-z
Souri D (2008) J Phys D Appl Phys 41:105102
Souri D, Elahi M (2007) Phys Scr 75(2):219
Souri D, Elahi M, Yazdanpanah MS (2008) Cent Eur J Phys 6(2):306
Chowdari BVR, Kumari PP (1997) J Phys Chem Solids 58(3):515
Pal M, Hirota K, Tsujigami Y, Sakata H (2001) J Phys D Appl Phys 34:459
Sharma BK, Dube DC, Mansingh A (1984) J Non-Cryst Solids 65:39
Murugan GS, Ohishi Y (2004) J Non-Cryst Solids 341:86
Jayaseelan S, Muralidharan P, Venkateswarlu M, Satyanarayana N (2005) Mater Sci Eng B 118:136
El-Mallawany R, Abousehly A, Yousef E (2000) J Mater Sci Lett 19:409
El-Adawy A, El-Mallawany R (1996) J Mater Sci Lett 15:2065
Abdel-Kader A, El-Mallawany R, Elkholy MM (1993) J Appl Phys 73(1):71
El-Mallawany R (2000) Phys Status Solidi (a) 177:439
Sidkey MA, El-Mallawany R, Abousehly A, Saddeek YB (2002) Glass Sci Technol Glastech Ber 75:87
Turky G, Dawy M (2002) Mater Chem Phys 77:48
Scherer GW (1986) Relaxation in glasses and composites. Wiley, New York
Gerlach E, Grosse P (eds) (1979) The physics of selenium and tellurium. Springer, Berlin
Bishop SG, Strom U, Taylor PC (1975) Phys Rev Lett 34:1346
Donohue J (1974) The structure of the elements. Wiley, New York
Moynihan CT, Easteal AJ, Wilder J, Tucker J (1974) J Phys Chem 78:2673
Elliott SR (1990) Physics of amorphous materials, 2nd edition edn. Longman Scientific & Technical, Essex
Avramov I, Guinev G, Rodrigues ACM (2000) J Non-Cryst Solids 271:12
Zhu D, Ray CS, Zhou W, Day DE (2011) Phys Chem Glasses (in press)
Sega K, Kuroda Y, Sakata H (1998) J Mater Sci 33:1303. doi:https://doi.org/10.1023/A:1004302431797
Lebrun N, Levy M, Soquet JL (1990) Solid State Ionics 40(41):718
Dhawan VK, Mansingh A (1982) J Non-Cryst Solids 51:87
Smith GW, Pinkerton FE, Moleski JJ (1999) Thermochim Acta 342:31
Vyazovkin S, Sbirrazzuoli N, Dranca I (2004) Macromol Rapid Commun 25:1708
Abu-Sehly Aa, Abu El-Oyoun M, Elabbar AA (2008) Thermochim Acta 472:25
Zhu D, Ray CS, Zhou W, Day DE (2003) J Non-Cryst Solids 319:247
Rocherull’e J, Matecki M, Delugeard Y (1998) J Non-Cryst Solids 238:51
Lasocka M (1976) Mater Sci Eng 23:173
Sinclair RN, Wrigth AC, Bachra B, Dimitriev YB, Dimitrov VV, Arnaudov MG (1998) J Non-Cryst Solids 232–234:38
El-Mallawany R, Abdel-Kader A, El-Hawary M, El-Khoshkhany N (2010) J Mater Sci 45:871. doi:https://doi.org/10.1007/s10853-009-4015-5
Mitrofanov YuP, Khonik VA, Granato AV, Joncich DM, Khonik SV (2011) J Appl Phys 109:073518
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souri, D. Investigation of glass transition temperature in (60 − x)V2O5–40TeO2−xNiO glasses at different heating rates. J Mater Sci 46, 6998–7003 (2011). https://doi.org/10.1007/s10853-011-5668-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-5668-4