Abstract
Thermal properties and crystallization of glasses from PbO–MoO3–P2O5 ternary system were studied in three compositional series (100 − x)[0.5PbO–0.5P2O5]–xMoO3 (A), 50PbO–yMoO3–(50 − y)P2O5 (B), and (50 − z)PbO–zMoO3–50P2O5 (C). Glass transition temperature, crystallization temperature, coefficient of thermal expansion, and dilatation softening temperature of the studied glasses were determined by differential thermal analysis and dilatometry. Crystallization products of annealed glass samples were investigated by X-ray diffraction and Raman spectroscopy. X-ray diffraction analysis of crystallized glasses revealed the formation of PbP2O6, Pb3P4O13, and PbMoO4 in the samples of the B series. In the series A and C in the samples with a high MoO3 content, crystalline compounds of Pb(MoO2)2(PO4)2 and (MoO2)(PO3)2, respectively, were identified. Raman spectra of crystalline samples confirmed the results of X-ray diffraction measurements and provided also information on thermal stability of glasses and formation of glass-crystalline phases in the studied glass series.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphate glasses with additions of tungsten oxide or molybdenum oxide offer prospective applications for their electrochromic properties and high ionic conductivity [1, 2]. Silver- and lithium-containing molybdato-phosphate glasses were investigated for their prospective applications as ionic conductors [3]. Phosphate glasses belong also among possible candidate materials for the vitrification of nuclear wastes with molybdenum because they possess high waste loading capacities [4, 5]. Therefore, study of their thermal behavior and the process of their crystallization are interesting for potential application of these glasses.
Chowdari et al. [3, 6, 7] reported thermal and conductivity studies of Ag2O–MoO3–P2O5 and Li2O–MoO3–P2O5 system glasses. Bih et al. [8] prepared (1 − x)NaPO3–xMoO3 glasses with x = 0–0.5 and studied their thermal properties, EPR and their structure from IR spectra. They found non-regular variation of the glass transition temperature, T g, with increasing MoO3 content with a maximum close to x = 0.2. Santagnelli et al. [9] studied also (1 − x)NaPO3–xMoO3 glass series, but they prepared glasses within the broader compositional region of 0 ≤ x≤0.7. They found also a maximum on the compositional dependence of T g values, but much higher at x = 0.45. Thermal properties of Li2O–MoO3–P2O5 and Na2O–MoO3–P2O5 glasses studied Bih et al. [10] in three compositional series within the glass forming region and gave the data on glass transition temperatures and crystallization temperatures.
Lead phosphomolybdate glasses were studied by Selvaraj and Rao [11] in two compositional series (80 − x)PbO–20P2O5–xMoO3 and (60 − x)PbO–40P2O5–xMoO3 with 20 and 40 mol% P2O5. They applied DSC technique for the determination of glass transition temperature and heat capacity of glasses in these two compositional series of the PbO–MoO3–P2O5 system. Our recent studies [12] devoted to the study of structure of glasses in the PbO–MoO3–P2O5-ternary system by Raman and 31P NMR spectroscopy showed on the formation of MoO6 octahedral units in most glasses of the system, but also on the existence of tetrahedral MoO4 units in glasses with a high MoO3 content and a low P2O5 content.
In this work, thermal behavior and crystallization of glasses in the PbO-MoO3-P2O5 ternary system were investigated in three compositional series starting from Pb(PO3)2 and containing ≤50 mol% P2O5.
Experimental
Glasses of the PbO–MoO3–P2O5 system were prepared from reagent grade PbO, H3PO4 and MoO3 in batches of 25 g. In the first stage, the reaction mixture was heated slowly in a platinum crucible up to 600 °C with the final calcination at the maximum temperature for 2 h to remove water. After the calcination the reaction mixture was heated up to the 800–1000 °C, according to the composition. After 20 min heating at the maximum temperature, the melt was cooled by pouring into a graphite mold. The obtained glasses were separately annealed for 15 min at a temperature close to their T g and then slowly cooled to room temperature. Glass composition was checked by weighing and also by chemical analysis. The changes in the MoO3 content were lower than 0.5 mol%. The amorphous character of the obtained glasses was checked by X-ray diffraction analysis.
Thermal behavior of glasses was studied with the DTA 404 PC (Netzsch) operating in the DSC mode at the heating rate of 10 °C min−1. From the obtained, DTA curves glass transition transformation temperature, \( T_{\text{g}}^{\text{a}} \) , was determined as the onset of the change in the heat capacity, C p, in the glass transformation region and crystallization temperature, T c, of the glasses was determined as the onset of the exothermic crystallization peak. The measurements were carried out on powder samples with a mean diameter 8–10 μm placed in silica crucibles under an atmosphere of N2. The thermal expansion coefficient, α, the glass transition temperature, T g, and dilatation softening temperature, T d, were measured on bulk samples with dimensions of 25 × 5×5 mm using dilatometer DIL 402 PC (Netzsch). From the obtained dilatation curves, the coefficient of thermal expansion, α, was determined as a mean value in the temperature range of 100–200 °C, the glass transition temperature, \( T_{\text{g}}^{\text{b}} \), was determined from the change in the slope of the elongation versus temperature plot and the dilatation softening temperature, T d, was determined as the maximum of the expansion trace corresponding to the onset of viscous deformation. The dilatometric measurements were carried out in air at the heating rate of 5 °C min−1.
Glass crystallization was studied by annealing the powdered glass samples at temperatures slightly above their crystallization peak for 2 h. Crystalline products were identified by X-ray diffraction analyses of annealed samples on Bruker D8 Advance X-ray diffractometer with the application of PDF 4 database for the determination of crystalline products.
Annealed samples were also analyzed by Raman spectroscopy for the identification of glass-crystalline phases. The Raman spectra were measured at room temperature using Horiba Jobin-Yvon LabRam HR spectrometer. The spectra were recorded in back-scattering geometry under excitation with He–Ne laser radiation (632.8 nm) at a power of 12 mW. The spectral slit width was 1 mm, exposure time was 3 s and accumulation number was 10.
Results and discussion
We have prepared 14 glass samples of the PbO–MoO3–P2O5 ternary system by slow cooling of the corresponding melt in air to room temperature. Their amorphous character was confirmed by X-ray diffraction. We have studied changes in their thermal properties in three compositional series (100 − x)[0.5PbO–0.5P2O5]–xMoO3 (series A), 50PbO–yMoO3-(50 − y)P2O5 (series B) and (50 − z)PbO–zMoO3–50P2O5 (series C). Their composition is shown in Table 1. Glass samples containing MoO3 were of blue color showing on the presence of Mo5+ species in the glasses which was confirmed by EPR spectroscopy. The EPR studies also showed that the ratio of Mo5+/Mototal in most samples is lower than 0.9%.
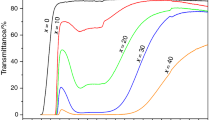
DSC curves obtained for the glass series (A) are shown in Fig. 1. The values of glass transition temperature, \( T_{\text{g}}^{\text{a}} \) and crystallization temperature T c, determined from DSC curves are given in Table 1. We have determined glass transition temperature by two methods. From DSC curves, \( T_{\text{g}}^{\text{a}} \) values were determined as the intersection between the extrapolated line of the glass heat flow before T g and the extrapolated line of the changing heat flow in the glass transition region, according to the procedure proposed by Yue [13]. The values of \( T_{\text{g}}^{\text{a}} \) determined by this procedure from the onset of the change in heat flow on DSC curves in the glass transformation region are very close to \( T_{\text{g}}^{\text{b}} \) values obtained from the change in the slope of the elongation versus temperature plot of the dilatometric measurements in agreement with [13]. In Table 1, the values of both T g are given. On some DSC curves also endothermic peaks of melting of the crystallized glasses can be seen with their characteristic temperature T m, usually determined as the onset of the endothermic peaks.
DSC curves of the glass series (100 − x)[0.5PbO–0.5P2O5]–xMoO3 (A series). The values of T g and T c are given in Table 1
In the (A) series (100 − x)[0.5PbO–0.5P2O5]–xMoO3 (0 < x<70) under slow cooling in air, we prepared glasses in the compositional range of 0–70 mol% MoO3. Their glass transition temperature, according to DTA measurements, increases with increasing MoO3 content from 309 °C with increasing MoO3 content and reaches a maximum of 420 °C at the glass with 50 mol% MoO3 and then decreases. The T g values obtained from dilatometric curves (Table 1) are close to those determined from DSC curves as the onset of the change in heat flow in the glass transition region (Fig. 1). As the changes in ΔC p within the glass transition region on the DSC curves of glasses with x = 40–70 mol% MoO3 in Fig. 1 are too small, the magnified part of the DSC curves is shown in Fig. 2 together with an example of the determination of T g. Most glasses crystallize on heating as manifested on DSC curves in Fig. 1 by the presence of an exothermic crystallization peak. Crystallization temperatures determined as the onset of the crystallization peak are also given in Table 1. Most thermally stable glasses in this series are those containing 20–30 mol% MoO3 which do not crystallize on heating. Coefficient of thermal expansion decreases with increasing MoO3 content from 17.9 ppm/°C down to 13.7 ppm/°C at the glass with 50 mol% MoO3 and then slowly increases. Glass transition temperature in the series (B) 50PbO–yMoO3-(50 − y)P2O5 also increases with increasing MoO3 content and reaches a maximum of 420 °C at the glass with 30 mol% MoO3, whereas thermal expansion coefficient decreases but goes down only to 15.9 ppm/°C (see Table 1). In the glass series (C) (50 − z)PbO–zMoO3–50P2O5, the T g and T c values increase within the range of 0–-20 mol% MoO3 and then decrease, whereas thermal expansion coefficient goes rapidly down.
In order to indentify products of crystallization of the studied glasses, we have milled the glass samples and heat treated the obtained powder for 2 h at temperatures slightly above their corresponding crystallization peaks. The annealing temperatures are given in Table 2 together with the composition of crystalline phases identified by X-ray diffraction analysis in all three glass series. For the identification of the results of crystallization, we have applied also the Raman spectroscopy and the obtained Raman spectra are shown in Figs. 3, 6, and 7.
Raman spectra of the annealed (100 − x)[0.5PbO–0.5P2O5]–xMoO3 samples (A series). Crystalline phases identified by XRD in the annealed samples are given in Table 2
In the glass series (A) (100 − x)[0.5PbO–0.5P2O5]–xMoO3 in samples with 0–10 mol% MoO3 the only crystalline phase identified in crystallization products by X-ray diffraction was lead metaphosphate PbP2O6. Raman spectra also revealed the presence of crystalline lead metaphosphate with characteristic strong vibrational bands of 1150 and 670 cm−1 [14]. Raman spectrum of the glass with x = 10 mol% MoO3 (Fig. 3) shows within the region of 820–980 cm−1 also the presence of two broad Raman bands characteristic of the presence of molybdate structural units in the glassy phase [15, 16]. It means that the annealed sample contains one crystalline phase (PbP2O6) and one glassy phase containing MoO3. Glasses with x = 20–30 mol% MoO3 are thermally stable and do not reveal any crystallization peak on DSC curves on heating with the heating rate of 10 °C/min. Also their annealing at three different temperatures did not result in their crystallization as revealed by X-ray diffraction and their Raman spectra (Fig. 3) reveal only the presence of glassy phase with two dominant broad bands within the region of 850–970 cm−1 ascribed to vibrations of molybdate units [15, 16]. DSC curve on heating the sample with x = 40 mol% MoO3 does not show any visible crystallization peak, but shows a weak endothermic melting peak at 696 °C which reveals a partial crystallization of the glassy sample. X-ray diffraction analysis of annealed samples with x = 40 and 50 mol% MoO3 identified the presence of one crystalline phase of the composition of Pb(MoO2)2(PO4)2. Its composition corresponds exactly to the glass with x = 50 mol% MoO3 and on its DSC curve, there is one exothermic peak of crystallization and one endothermic melting peak. In the annealed samples of glasses with x = 60 and 70 mol% MoO3 X-ray diffraction found the presence of the compound Pb(MoO2)2(PO4)2 and crystalline MoO3. The DSC curve of the sample with x = 60 mol% MoO3 (Fig. 1) shows two melting peaks which corresponds to the presence of two crystalline phases. Raman spectra of these samples (Fig. 3) are in good agreement with the results of XRD.
We have devoted our attention also to the compound of Pb(MoO2)2(PO4)2. Its structure was determined by Masse et al. [17]. This compound is interesting for the study due to its ability to form glassy state. As the chemical composition of the melt and the corresponding crystalline phase are the same, it is possible to evaluate the processes of crystallization and melting. On the bases of several DTA measurements with the heating rate within the range of 5–20 °C/min in the first step the activation energy of crystallization was determined as \( E_{\text{c}}^{*} = 30 8\pm 3 \) kJ/mol (3.19 ± 0.03 eV) with the application of Kissinger method [18]. In the second step, we have applied obtained data also for the evaluation of an appropriate kinetic model of the crystallization process. We have found that the crystallization kinetics can be described by the Šesták-Berggren model SB(m, n) [19] with parameters of m = 0.88 ± 0.18 n = 1.52 ± 0.31.
We have determined also melting temperature of the Pb(MoO2)2(PO4)2 compound as T m = 706 ± 3 °C, peak temperature of the melting process was T p,m = 747 ± 7 °C. Melting enthalpy was determined as ΔH m = 39.7 ± 1.4 J/g. We have also evaluated glass transformation process in the glass of this composition. Glass transition temperature increases with increasing heating rate from 417 °C (5 °C/min) to 424 °C (20 °C/min). Activation energy of glass transition process was evaluated also with the application of Kissinger method [18] as \( E_{{T_{\text{g}} }}^{*} = 4 9 6 \pm 3 9 \) kJ/mol (5.14 ± 0.51 eV). The change in the isobaric heat capacity at the glass transition is ΔC p = 0.138 J/(g K).
In Fig. 4, there is a comparison of Raman spectra of polycrystalline compound Pb(MoO2)2(PO4)2 and the corresponding glass. Raman spectra of the polycrystalline compound are in a good agreement with the tabular data of Raman spectra given by Isaac et al. [20]. From Fig. 4, it is evident that the main features of the Raman spectrum of the glass can be obtained by broadening the bands of the Raman spectrum of the crystals corresponding to fluctuations of bonding angles and bond lengths in the disordered glassy network. From the similarity of basic spectral features of both spectra, we can assume that the short-range order in the glass is similar to that in the crystal Pb(MoO2)2(PO4)2 and that basic structural units in both phases are probably the same.
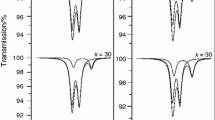
In the (B) series of glasses 50PbO–yMoO3–(50 − y)P2O5 under slow cooling in air, we prepared glasses in the compositional range of 0–30 mol% MoO3. Their glass transition temperature increases also with increasing MoO3 content by 111 °C as shown on DSC curves in Fig. 5. All glasses crystallize on heating and the difference T c − T g is the highest at the glass with y = 10 mol% MoO3. Crystallization temperatures determined as the onset of crystallization peak are given in Table 1. X-ray diffraction revealed various products formed in the annealed samples (Table 2). Crystalline PbP2O6 is formed only in the crystallized pure lead metaphosphate glass, whereas in the sample with y = 10 mol% MoO3 the presence of lead tetraphosphate Pb3P4O13 was identified. Lead diphosphate Pb2P2O7 is formed in the annealed sample with y = 20 mol% MoO3 together with the compound Pb(MoO2)2(PO4)2 identified also in the series A. The XRD analysis of the crystallized sample with y = 30 mol% MoO3 identified lead molybdate PbMoO4 as the only crystalline phase. It means that some lead phosphate remained in the glassy state here. Raman spectra of the annealed samples (Fig. 6) are in good agreement with the results of XRD analysis. In the sample with x = 10 mol% MoO3 molybdate phase remains amorphous as evidenced by a broad Raman band within the region of 850–970 cm−1. The presence of crystalline phase Pb(MoO2)2(PO4)2 is evident in the annealed simple with y = 20 mol% MoO3, where characteristic Raman bands are similar to those shown in Fig. 5.
DSC curves of the glass series 50PbO–(50 − y)P2O5–yMoO3 (B series). The values of T g and T c are given in Table 1
Raman spectra of the annealed 50PbO–(50 − y)P2O5–yMoO3 samples (B series). Crystalline phases identified by XRD in the annealed samples are given in Table 2
In the (C) series of glasses (50 − z)PbO–zMoO3–50P2O5, we prepared glasses with z = 0–30 mol% MoO3. Thermal analysis showed here also an increase in the glass transition temperature with increasing MoO3 content, but both T g and T c values at the glass with z = 30 mol% MoO3 are lower. Previous study [11] revealed in this glass a high content of Mo5+ ions, the presence of which according to Flower et al. [21] weakens the glass network, as Mo5+ ions occupy network-modifying positions in the glass structure and decrease the rigidity of the glass network. This conclusion could explain decreased values of T g in the glass 20PbO–50P2O5–30MoO3. Coefficient of thermal expansion decreases here with increasing MoO3 content as shown in Table 1.
XRD analysis identified in the annealed samples with z = 0–20 mol% MoO3 only PbP2O6 as the crystalline phase, whereas molybdate phase remained amorphous as evidenced from the Raman spectra (Fig. 7) of these samples by the presence of two broad bands within the range of 850–970 cm−1. In the sample with z = 30 mol%, MoO3 crystalline phase of the composition (MoO2)(PO3)2 was identified by XRD analysis. This result is in agreement with the Raman spectra of annealed samples (Fig. 7), where the characteristic band of the compound (MoO2)(PO3)2 at 984 cm−1 was observed. This dominant Raman band corresponds to the dominant band in the Raman spectrum of the (MoO2)(PO3)2 compound published by Morgan and Magruder [22]. The structure of crystalline (MoO2)(PO3)2 contains MoO6 octahedra and chains of PO4 units [23]. Annealed sample with z = 30 mol% MoO3 contains a part of the sample in the glassy state as evidenced in its Raman spectrum by the presence of a broad Raman bands within the range of 850–1000 cm−1.
Raman spectra of the annealed (50 − z)PbO–50P2O5–zMoO3 samples (C series). Crystalline phases identified by XRD in the annealed samples are given in Table 2
Conclusion
Study of thermal behavior and crystallization of PbO–MoO3–P2O5 ternary system glasses by combined techniques of thermal analysis, X-ray diffraction, and Raman spectroscopy showed to be very effective from the point of characterization of annealed samples. DSC studies showed on a good thermal stability of glasses of the A series with 20–30 mol% MoO3, which do not crystallize on heating or annealing. Basic characterization of crystalline products by XRD is naturally most important. Nevertheless, Raman spectroscopy is able not only to verify obtained crystalline products, but also to detect the existence of glassy phases in the samples which do not crystallize completely. Crystallization of some glassy samples showed on the formation of crystalline lead molybdato-phosphate compound Pb(MoO2)2(PO4)2 in some glasses with a high MoO3 content. We assume that some structural motifs of this compound are present in the corresponding glasses. In the P2O5-rich crystallized samples, another compound (MoO2)(PO3)2 was identified, the structure of which contains MoO6 octahedra and PO4 tetrahedra, and we are convinced that short-range order in crystalline and glassy phases is the same.
References
Zakis YR, Lusis AR, Lagzdons YL (1982) J Non-Cryst Solids 47:267
Studer F, Lebail A, Raveau B (1986) J Solid State Chem 63:414
Chowdari BVR, Gopalakrishnan R, Tang SH, Kuok SH (1988) Solid State Ion 28–30:704
Pinet O, Dussossoz JL, David C, Fillet C (2008) J Nucl Mater 377:307
Chourasia R, Shrivastava OP, Wattal PK (2009) J Alloys Comp 473:579
Chowdari BVR, Tan KL, Chia WT, Gopalakrishnan R (1990) Solid State Ion 40–41:684
Chowdari BVR, Tan KL, Chia WT, Gopalakrishnan R (1991) J Non-Cryst Solids 128:18
Bih L, Nadiri A, El Amraoui Y (2005) J Phys IV Fr 123:165
Santagneli SH, de Araujo CC, Strojek W, Eckert H, Poirier G, Ribeiro SJL, Messadeq Y (2007) J Phys Chem B 111:10109
Bih L, Nadiri A, Aride J (2002) J Therm Anal Calorim 68:965
Selvaraj U, Rao KJ (1988) J Non-Cryst Solids 104:300
Koudelka L, Rösslerová I, Holubová J, Mošner P, Montagne L, Delevoye L (2011) J Non-Cryst Solids. doi:https://doi.org/10.1016/j.jnoncrysol.2011.03.006
Yue Y-Z (2008) J Non-Cryst Solids 354:1112
Bobovich YaS (1962) Opt Spektrosk 13:459
Chowdari BVR, Tan KL, Chia WT (1993) Mater Res Soc Symp Proc 293:325
Šubčík J, Koudelka L, Mošner P, Montagne L, Trickot G, Delevoye L, Gregora I (2010) J Non-Cryst Solids 356:2509
Masse R, Averbuch-Pouchot MT, Durif A (1985) J Solid State Chem 58:157
Kissinger HE (1957) Anal Chem 29:1702
Šesták J, Berggren G (1971) Thermochim Acta 3:1
Isaac M, Jayasree V, Suresh G, Nayar VU (1992) Ind J Phys B 66:65
Flower GL, Baskaran GS, Reddy MS, Veeraiah N (2007) Phys B 2393:61
Morgan SH, Magruder RH (1990) J Am Ceram Soc 73:753
Kierkegaard P (1961) Ark Kemi 18:521
Joint Committee on Powder Diffraction Standards, International Centre of Diffraction Data, Swarthmore, PA, USA
Acknowledgements
The authors are grateful for the financial support from the research project No. P106/10/0283 of the Grant Agency of Czech Republic and from the research project No. 0021627501 of the Ministry of Education of Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rösslerová, I., Koudelka, L., Černošek, Z. et al. Thermal properties and crystallization of PbO–MoO3–P2O5 glasses. J Mater Sci 46, 6751–6757 (2011). https://doi.org/10.1007/s10853-011-5631-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-5631-4