Abstract
The effect of water vapour was studied on a nickel-based SY 625 alloy oxidized at 900, 1000 and 1100 °C under dry and wet conditions. It appears that H2O has little effect on the oxidation rate and scale composition after 48 h. The outer scale is composed of chromia Cr2O3. At 900 and 1,000 °C, NbNi4 and Ni3Mo intermetallics are found at the oxide/alloy interface. At 1,100 °C, the scale is composed of an outer chromia scale and an internal CrNbO4 subscale. At this temperature the oxide scale morphology differs between dry and wet conditions. Under dry conditions the oxide scale appears to be compact but the external part of the scale partially spalled of during cooling. The oxide scales formed under wet conditions show porosities spread inside the scale and the chromia grain size is smaller. At 1,100 °C scale spallation is observed under dry conditions due void accumulation in the middle part of the scale. Under wet conditions the uniform distribution of the porosities inside the scale leads to a better scale adherence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous industrial processes correspond to corrosive environments containing water vapour. In fuel cells (SOFC) [1], waste incineration, engines, aeronautic and supercritical boilers now being developed for power plant [2] and combustion systems [3], the choice of the materials must take into account their high temperature corrosion resistance under wet conditions. On FeCrAl alloys at 1,000 °C water vapour appears to reduce the mass gain and modifies the scale nature favouring iron and chromium presence inside the scale leading to a bad corrosion protection [4, 5]. The effect of water vapour on transient alumina transformation was also observed on Fe3Al intermetallics [6].
On stainless steels, chromium remains the essential alloying element for all these steels, provided a protective Cr2O3 chromia layer is formed. On pure chromium it has been demonstrated that water vapour mainly acts on the chromia grain size and scale plasticity by preventing scale cracking [7]. Nevertheless, the literature shows that most iron–chromium alloys show breakaway oxidation under wet conditions due to iron oxidation [8–18]. Recently, it has been shown that the addition of water vapour to Ar/O2 or Ar/H2 atmospheres at 700 °C, accelerates the onset of breakaway oxidation kinetics for Fe9Cr alloys and promotes internal chromium oxidation. On higher chromium containing FeCr alloys, the water vapour effect is not significant due to the large chromium reservoir insuring a continuous growth of Cr2O3 [19].
In order to avoid all fast growing corrosion products induced by iron oxidation under wet oxidizing conditions, nickel-based alloys Inconel 625 or SY 625 could be employed due to the very low iron content of the alloy. In turbine blade materials developments, operating temperatures are high and combustion gases contain water vapour. The current materials used are nickel-based superalloys, which possess very high temperature strength. This is important to withstand the important centrifugal forces generated by the blade rotational speeds in the case of jet engines. In dry high temperature oxidation conditions some literature data are proposed [20–27].
Khalid et al. showed that the oxidation of the Inconel 625 at 1,000 °C leads to a Cr2O3 external scale adherent and uniform on rolled specimens. Lower weight gain, breaking and spallation of the oxide appeared on deformed specimens due to the dynamic changes occurring in the metallic deformed substructure [23]. Under cyclic oxidation conditions, alloy Inconel 625 showed a strong resistance at 900 °C. At 1,000 °C, chromium loss led to chromium depletion due to a re-oxidation effect. Then, the chromia scale loses its protective properties and alloy Inconel 625 exhibits a lower cyclic oxidation resistance [26]. On Inconel 625 erosion-corrosion at high temperatures show that oxidation is enhanced during erosion. It was caused by inward transport of oxygen through cracks and other defects created by the particle bombardment. The greatest wastage rates were measured at 700 °C and growth of oxide nodules are observed at the oxide/metal interface causing protruding oxide flakes, which are chipped away [20, 21].
On chromia-forming nickel-based alloys, very limited information is available about oxidation in water vapour containing atmospheres.
For the nickel-base system, previous studies indicate that a little increase in the growth rates is observed when water vapour is present in the gaseous environment [27–29]. Water vapour does not drastically modify the scale composition compared to dry environments [30, 31]. Nevertheless, it is generally shown that the adherence is improved and a more porous chromia scale is formed under wet conditions [32, 33]. The oxide grain size can also be affected by moisture under high temperature oxidation conditions. It is important to mention that the major difference between Fe-base and Ni-base alloys suggests that in the case of the Fe-base alloys, water vapour induces an acceleration of iron oxidation at high water vapour partial pressure and high temperatures. As long as nickel-base alloys do not contain iron in their composition it is not expected to observe a breakaway during oxidation which could be attributable to iron oxidation.
In order to better understand the oxidation mechanism under wet environments, this work will focus on the isothermal oxidation behaviour of a nickel-based alloy SY 625 between 900 and 1,100 °C. Structural analysis will allow us to identify the corrosion products, the scale morphology and to correlate the alloy behaviour with the main alloying elements.
Experimental details
The material used in the present study is a SY625 alloy provided by ArcelorMittal Imphy (France). The alloy composition is given in Table 1. The specimens are 1.6 mm thick and show a total surface area of around 5 cm2. The specimens were polished on SiC paper up to the 800 polishing grade. Then, they were washed with ethanol and finally dried before isothermal oxidation between 900 and 1,100 °C. The experiments were conducted in a Setaram Setsys TGA microthermobalance, adapted for oxidation during 48 h, in dry (0.3 vol.% H2O) or wet air (7.5 vol.% H2O) gas mixtures generated by a Setaram Wetsys humidifier. The carrier gas is composed of synthetic air (AlphaGaz 1). The flowing rate is 1.2 L/h under a total 1 atm pressure. All the connecting tubes between the different parts of the equipment and the cooling water of the thermobalance are maintained at 75 °C using heating wires or transfer lines in order to avoid any water condensation. The heating rate is 60 °C/min in argon. Isothermal oxidation starts when switching to the gaseous oxidizing environment. The cooling rate corresponds to 60 °C/min in argon.
The in situ characterisation of the oxide scales formed under dry condition was carried out in a high temperature MRI chamber adapted on an X-ray Philips X’ PERT MPD diffractometer (copper radiation, \( \lambda_{K\alpha } \) = 0.154 nm). Diffraction peaks will be considered as significant if their relative intensity is greater than 10% of the intensity reported on their corresponding International Center for Diffraction Data (ICDD) files. The X-ray diffraction (XRD) patterns are registered every hour in the 20–80 2Θ degrees range at 900, 1000 or 1100 °C. After wet oxidation tests, XRD were performed after cooling to room temperature, on specimens oxidized during 2, 5, 24 or 48 h. The chromia grain size has been estimated by XRD and with the help of the Visual Crystal Software from Corporation Software using the refinement method proposed by Rietveld [34]. To determine the Pseudo-Voight curve, Visual Crystal calculates polynomial fitting equations representing the half width of the peaks as a function of their angular positions using least squares method [35]. The oxide scale surface and cross-section morphologies and chemistry were investigated by using a scanning electron microscopy (SEM) JEOL JSM-6400F equipped with an energy dispersive X-ray spectroscopy analyser (EDS).
Results
Oxidation kinetics
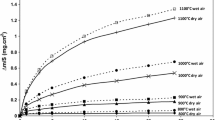
Kinetic curves obtained with SY 625 specimens oxidized, during 48 h at 900, 1000 and 1100 °C, in flowing air, are presented on Fig. 1. After oxidation at 900 and 1,000 °C, little scale spallation is observed during cooling to room temperature. At 1,100 °C, spallation is more important after the 48 h oxidation tests under dry conditions. Weight gain curves show that the oxidation rate follows a parabolic behaviour. The parabolic rate constants are, respectively: k p = 3.2 × 10−13, 7.4 × 10−12 and 5.25 × 10−11 g2 cm−4 s−1 at 900, 1000 and 1100 °C.
Under wet conditions, the mass gain versus time curves for SY 625 specimens oxidized, during 48 h at 900, 1000 and 1100 °C, in a 7.5 vol.% H2O/air gas mixture are presented on Fig. 1. After oxidation at 900, 1000 and 1100 °C little scale spallation is observed after cooling to room temperature. The mass gain curves show that the oxidation rate follows a parabolic behaviour. The parabolic rate constants are, respectively: k p = 3.8 × 10−13, 7.96 × 10−12 and 6.23 × 10−11 g2 cm−4 s−1 at 900, 1000 and 1100 °C.
The parabolic behaviour was followed in this temperature range. This permits the calculation of the parabolic rate constants k p at each temperature under dry and wet conditions. The parabolic rate constants k p are reported in Table 2. Kinetic results clearly show that water vapour does not influence the oxidation rate of the SY 625 alloy in our experimental conditions and within experimental errors.
Corresponding to dry and wet conditions, activation energies (E a) were calculated from the slope of the Arrhenius plots of the k p values. The calculated activation energy are, respectively, E a = 316 and 308 ± 10 kJ mol−1 under dry and wet conditions.
XRD results
XRD on sample oxidized in dry air
Oxide scales formed on the SY 625 alloy were analyzed by in situ XRD on the metallic substrate at the testing temperature after 48 h (Fig. 2). In dry conditions, the X-ray diffraction patterns show the presence of Cr2O3 (ICDD 38-1479); NbNi4 (ICDD 20-0787) and Ni3Mo (ICDD 17-0572) after oxidation at 900 and 1,000 °C. At 1,100 °C the scale structure is different. It is composed of chromia and CrNbO4 (ICDD 34-0366). It has also been observed that under dry conditions the oxide scale composition does not change during the 48 h oxidation test. Chromia and the intermetallics NbNi4 and Ni3Mo are formed from the first hour of oxidation and remains all along the test at 900 and 1,000 °C. At 1,100 °C, NbNi4 and Ni3Mo are not detected anymore. At this temperature, Cr2O3 and CrNbO4 are formed from the beginning of the test. In dry air, the comparison of the XRD results obtained in situ at the testing temperature and after cooling to room temperature show that the same oxides are detected and that no phase transition occurred during cooling. These results indicate that a comparison between XRD results under dry and wet conditions is allowed.
XRD on samples oxidized in wet air
Oxide scales formed under wet conditions (7.5 vol.% H2O) on the SY 625 alloy were analyzed by XRD on the metallic substrate after cooling to room temperature (Fig. 2). The X-ray diffraction patterns show the presence of Cr2O3 (ICDD 38-1479); NbNi4 (ICDD 20-0787) and Ni3Mo (ICDD 17-0572) after oxidation at 900 and 1,000 °C. At 1,100 °C the scale is composed of chromia and CrNbO4 (ICDD 34-0366). X-ray diffraction results show that 7.5 vol.% water vapour does not influence the oxide scale composition.
Oxide scale morphology
Surface analysis
For each temperature (900, 1000 and 1100 °C), the surface morphology of the scale formed on the SY 625 alloy oxidized during 48 h, in dry or wet conditions, is presented on Fig. 3. No scale spallation was observed on the oxide surface obtained at 900 and 1,000 °C. Chromia grain size measurements by XRD show that at 900 and 1,000 °C there is no detectable difference between dry and wet conditions. The mean chromia grain size is about 4 μm at 900 °C and 5 μm at 1,000 °C. At 1,100 °C results show that the mean chromia grain size is 5 μm under dry conditions but 20% lower under wet conditions (4 μm). It appears that the effect of water vapour on the chromia grain size is noticeable at high temperature (1,100 °C) but not at the lower temperatures (900–1,000 °C). At 1,100 °C, under dry conditions, 25% of the scale spalled off during cooling. In dry air, the oxide scale is composed of two subscales. The external chromia scale partially spalled off whereas the internal subscale remains adherent to the surface. EDS analysis of the sample surface shows the presence of chromium and oxygen in the outer subscale (Cr2O3). EDS exhibits the presence of oxygen, chromium and niobium in the inner subscale composed of Cr2O3 and CrNbO4. Under wet conditions the scale is more adherent, the only spalled area is shown on (Fig. 4). The oxide scale is composed of an outer chromia scale and an inner CrNbO4 subscale. When the external chromia scale spalled off, the internal CrNbO4 subscale remains on the surface.
Cross-section morphology
Cross-section micrographs were carried out in order to estimate the scale morphology and to identify the elements incorporated in the oxide scale under dry and wet conditions.
After 48 h oxidation at 900 °C in dry air, the scale is adherent (Fig. 5). EDS analysis show the presence of an outer chromia scale and an internal metallic part constituted by intermetallics NbNi4 and Ni3Mo (identified by XRD). Under wet conditions the chromia scale is well adherent on the surface. NbNi4 and Ni3Mo are also located at the alloy/oxide interface (Fig. 6).
After oxidation at 1,000 °C, in dry air, a compact chromia scale is formed (Fig. 7). Intermetallics NbNi4 and Ni3Mo appeared at the oxide/alloy interface. The chromia scale is adherent on the specimen surface. Some internal oxidation appears as chromia pegs inside the metallic matrix. With 7.5 vol.% water vapour in the gaseous environment, NbNi4 and Ni3Mo are also located at the oxide/alloy interface (Fig. 8). Under wet conditions, the chromia scale appears to be more porous.
After oxidation at 1,100 °C, under dry and wet conditions, some parts of the oxide scale spalled off during cooling.
Under dry conditions, Fig. 9 shows that the scale is about 8 μm thick whereas the scale is 16 μm thick under wet conditions. In fact under dry conditions the external part of the chromia scale partially spalled off during cooling. This is due to void accumulation in the middle of the chromia scale during the oxidation process in dry air. The cross-section only shows the remaining adherent part of the scale composed of an outer Cr2O3 scale and the inner continuous CrNbO4 subscale.
After 48 h oxidation in wet air, Fig. 10 exhibits a 16 μm thick chromia scale. The oxide scale is adherent under this condition. Compared to dry conditions, porosities are distributed in all the scale thickness and not accumulated locally inside the chromia scale. The internal oxide subscale is composed of CrNbO4.
Discussion
Kinetic aspects
Kinetic results show that the parabolic behaviour is followed at 900, 1000 and 1100 °C under dry and wet 7.5 vol.% H2O/air gas mixtures. Figure 1 shows that water vapour has no important effect on the oxidation rate. The calculated parabolic rate constants are very similar under dry and wet conditions (Table 2). Our results are in accordance with the study of other authors which have proposed that kinetics of oxidation on nickel-base systems were not strongly affected by the presence water vapour during short-term tests at temperatures up to 1,100 °C [27–31].
Chromium appears to be an alloying element leading to the protective Cr2O3 chromia layer formation during the SY 625 alloy oxidation. Nevertheless, the literature shows that many FeCr alloys show breakaway oxidation under wet condition due to the chromium depletion and iron oxidation at the scale/alloy interface [8–18]. It has been proposed that the Cr/Fe ratio of the oxide film increased with time and temperature but was lower in H2O-grown oxides compared to air-grown oxides [36]. Owing to the very low iron amount in the SY 625 alloy, no iron oxidation and no breakaway corrosion is observed within the 48 h oxidation duration.
Some authors demonstrated by means of a mass spectrometry that during the corrosion of steels in steam atmospheres, CrO2(OH)2 was identified as a volatile species [37, 38]. No evidence for the volatilisation of chromia was reported on a Ni–25Cr–0.01Y alloy when oxidized in air + 5 vol.% H2O at 950 °C for long exposures [39].
The calculated activation energies are, respectively, E a = 316 and 308 ± 10 kJ mol−1, respectively, under dry and wet conditions. These values are higher to the one generally encountered in the literature when Cr2O3 is formed on chromium or alloys in this temperature range [7, 40]. This higher activation energy is related to the fact that the scale is not only composed of chromia in the whole temperature range. At 900 and 1,000 °C, SEM cross-section observations and XRD results show that the scale was mainly composed of an external Cr2O3 scale and NbNi4–Ni3Mo intermetallics located at the internal interface. At 1,100 °C, oxidation leads of an external chromia layer and an internal CrNbO4 subscale. This indicates that in our temperature range 900–1,100 °C, even though the parabolic behaviour is followed, the scale structure is different and the diffusion process is probably modified when the chromia scale is not acting alone as a diffusion barrier at 1,100 °C. Then, the calculated activation energies are not representative of a diffusion process in a pure chromia scale.
Effect of water vapour on the oxide scale structure
XRD results show that the oxide scales formed under dry or wet conditions (7.5 vol.% H2O) on the SY 625 alloy is composed of Cr2O3; NbNi4 and Ni3Mo after oxidation at 900 and 1,000 °C. At 1,100 °C the scale is mainly composed of Cr2O3 and CrNbO4. X-ray diffraction results show that 7.5 vol.% water vapour does not show an influence on the oxide scale phase composition. In our study no nickel containing oxides are detected on the SY 625 alloy contrary to what was observed on other nickel-based substrates [41–45]. The scale composition observed is also not totally in accordance with the study of Khalid [23], which has found Cr2O3 but also MoO2 after an Inconel 625 oxidation at 1,000 °C.
After oxidation at 1,100 °C, NbNi4 is not detected anymore and the oxidation of niobium leads to the formation of CrNbO4. Ni3Mo is also not detected by XRD. This high temperature induces the molybdenum oxidation and MoO3 evaporation as proposed by other authors [24, 46–50]. It should be noticed that water vapour does not induce a modification in the scale composition at this temperature compared to dry conditions.
At the highest temperature, the chromia scale morphology appears to be more affected by water vapour. After 48 h oxidation in dry air, the scale is compact. Under wet conditions the chromia scale appears to be more porous. The void distribution inside the chromia scale is in accordance to what is generally proposed in the literature for chromia scales formed under wet conditions on Ni-based alloys [33, 37]. Under wet conditions, porous scales are also frequently observed on iron chromium alloys [8–18]. Norby [51] indicated that protons coming from water vapour dissociation may dissolve in oxide scales. It may enhance the oxygen transport in the form of hydroxide ion diffusion. The transport of protons can replace the outward transport of electrons during the scale growth. Henry et al. [52] proposed that pores are incorporated in the growing chromia scale whereas under dry conditions porosity was located at the scale/alloy interface. This is attributed to an increased cation vacancies concentration and thus higher outward chromium diffusion. At the same time there is an increase of the inward oxidant diffusion due to the presence of small OH− ions. The promoted outward cation diffusion process under wet condition can explain the fact that some nickel is detected at the external interface on Fig. 10.
Our study shows that at the highest temperature oxidation conditions (1,100 °C) the oxide grain size is reduced by 20% under wet conditions and the scale appears to be more adherent. This phenomenon was also observed by Jacob [7] when chromia scales are formed on pure chromium. Then, the good scale plasticity and adherence in moist gas mixture can be attributed to the smaller chromia grain size. It is proposed in the literature that in the case of high temperature oxidation, the presence of water refines the grains, but also introduces porosity. It is proposed by even if fluxes in chromia are much smaller than in iron oxides, when hydrogen or water vapour can access the scale interior oxygen mobility is effectively enhanced and voids grow [53].
At 900 and 1,000 °C, the good scale adherence on the SY625 alloy is attributed to a pegging effect. It could also be related to the presence of a high amount of molybdenum in the alloy. This phenomenon was also observed on another molybdenum-containing alloy (AISI 316L) oxidized under dry conditions at 900 °C [47].
Our results show that at 900 and 1,000 °C, molybdenum is found at the internal interface in a Ni3Mo compound. It should be noticed that low temperatures permit to avoid a MoO3 volatilisation from the steel surface as proposed by Wang [46]. Molybdenum-containing intermetallic Ni3Mo has been identified by XRD. The good scale adherence observed, can be due to the fact that molybdenum promotes the internal chromium oxidation leading to the pegging effect. At 1,100 °C scale spallation is observed under dry conditions due to decohesion of a part of the scale. Under wet conditions the uniform distribution of the porosities inside the scale and a smaller grain size lead to a better scale adherence.
Conclusion
The nickel-based SY 625 alloy has been oxidized at 900, 1000 and 1100 °C under dry and wet 7.5 vol.% H2O air gas mixture. Kinetic results show that the parabolic behaviour is followed and water vapour has no important influence on oxidation rate. X-ray diffraction results show that water vapour does not modify the oxide scale composition. At 900 and 1,000 °C the scale is composed of chromia Cr2O3 and two intermetallics (NbNi4 and Ni3Mo) are located at the oxide/alloy interface. At 1,100 °C the scale is composed of an outer chromia scale and an internal CrNbO4 continuous subscale. At 1,100 °C no more Ni3Mo is detected due to the molybdenum oxidation and MoO3 volatilisation. In situ XRD results under dry conditions show that the oxide scale composition does not change during the first 48 h oxidation. At 1,100 °C, SEM–EDS micrographs show that the morphology differs between dry and wet conditions. The oxide scale formed under dry air is compact. The oxide scales formed under wet conditions show porosities distributed inside the chromia scale. The good scale adherence observed under wet conditions at 900 and 1,000 °C is also attributed to a pegging effect of the chromia scale promoted by the presence of molybdenum. At 1,100 °C, scale spallation is observed under dry conditions due to decohesion of external parts of the chromia scale. Under wet conditions the uniform distribution of the porosities inside the scale leads to a better scale adherence.
References
Fontana S, Chevalier S, Caboche G (2009) J Power Sources 193:136
Viswanathan R, Sarver J, Tanzosh JM (2006) J Mater Eng Perform 15:255
Corrieu JM, Renaud L, Duret C, Cetre Y (2004) Mater Sci Forum 461–464:933
Buscail H, Heinze S, Dufour P (1997) J Chim Phys 94:553
Buscail H, Heinze S, Dufour P, Larpin JP (1997) Oxid Met 47:445
Chevalier S, Juzon P, Przybylski K, Larpin JP (2009) Sci Technol Adv Mater 10:1
Jacob YP, Haanappel VAC, Stroosnijder MF, Buscail H, Fielitz P, Borchardt G (2002) Corros Sci 44:2027
Zurek J, Michalik M, Schmitz F, Kern TU, Singheiser L, Quadakkers WJ (2005) Oxid Met 63:401
Schütze M, Renusch D, Schorr M (2005) Mater High Temp 22:113
Galerie A, Henry S, Wouters Y, Mermoux M, Petit JP, Antoni L (2005) Mat High Temp 22:105
Larring Y, Haugsrud R, Norby T (2003) J Electrochem Soc 150:B374
Yang Z, Xia G, Singh P, Stevenson JW (2005) Solid State Ionics 176:1495
Yang Z, Walker MS, Singh P, Stevenson JW, Norby T (2004) J Electrochem Soc 151:B669
Zeng XG, Young DJ (1994) Oxid Met 42:163
Peng X, Yan J, Zhou Y, Wang F (2005) Acta Mater 53:5079
Ehlers J, Young DJ, Smaardijk EJ, Tyagi AK, Penkalla HJ, Singheiser L, Quadakkers WJ (2006) Corros Sci 48:3428
Mikkelsen L, Linderot S (2003) Mater Sci Eng A361:198
Shen J, Zhou L, Li T (1997) Oxid Met 48:347
Othman NK, Othman N, Zhang J, Young DJ (2009) Corros Sci 51:3039
Norling R, Nylund A (2005) Oxid Met 63:87
Norling R, Olefjord I (2003) Wear 254:173
Saunders SRJ, Monteiro M, Rizzo F (2008) Prog Mater Sci 53:775
Khalid FA, Benjamin SE (2000) Oxid Met 54:63
Indacochea JE, Smith JL, Liyko KR, Karell EJ, Raraz AG (2001) Oxid Met 55:1
Birks N, Meier GH (1983) Introduction to high temperature oxidation of metals. Edward Arnold, New York
N’Dah E, Hierro MP, Borrero K, Perez FJ (2007) Oxid Met 68:9
England DM, Virkar AV (1999) J Electrochem Soc 146:3196
Rahmel A (1965) Corros Sci 5:815
England DM, Virkar AV (2001) J Electrochem Soc 148:A330
Hussain N, Shahid KA, Khan IH, Rahman S (1995) Oxid Met 43:363
Hussain N, Qureshi AH, Shahid KA, Chughtai NA, Khalid FA (2004) Oxid Met 61:355
Zurek J, Young DJ, Essuman E, Hänsel M, Penkalla HJ, Niewolak L, Quadakkers WJ (2008) Mater Sci Eng A477:259
Zurek J, Meier GH, Essuman E, Hänsel M, Singheiser L, Quadakkers WJ (2009) J Alloys Compd 467:450
Rietveld HM (1969) J Appl Cryst 2:65
Caglioti G, Paoletti A, Ricci FP (1958) Nucl Instr 3:223
Srisrual A, Coindeau S, Galerie A, Petit JP, Wouters Y (2009) Corros Sci 51:562
Perez FJ, Castaneda SI (2007) Surf Coat Technol 201:6239
Fryburg GC, Miller RA, Kohl FJ, Stearns CA (1977) J Electrochem Soc 124:1738
Holcomb GR, Alman DE (2006) Scripta Mater 54:1821
Chen JH, Rogers PM, Little JA (1997) Oxid Met 47:381
Zhou C, Yu J, Gong S, Huibin Xu (2002) Surf Coat Technol 161:86
Essuman E, Meier GH, Zurek J, Hänsel M, Norby T, Singheiser L, Quadakkers WJ (2008) Corros Sci 50:1753
Qiang Z, Rui T, Kaiju Y, Xin L, Lefu Z (2009) Corros Sci 51:2092
Wu Y, Narita T (2007) Surf Coat Technol 202:40
Tan L, Ren X, Sridharan K, Allen TR (2008) Corros Sci 50:3056
Wang X, Wang L-F, Zhu M-L, Zhang J-S, Lei M-K (2006) Trans Nonferrous Met Soc China 16:s676
Buscail H, El Messki S, Riffard F, Perrier S, Cueff R, Caudron E, Issartel C (2008) Mat Chem Phys 111:491
Hussain N, Shahid KA, Khan IH, Rahman S (1995) Oxid Met 43:363
Perez FJ, Otero E, Hierro MP, Gomez C, Pedraza F, De Segovia JL, Roman E (1998) Surf Coat Technol 108–109:127
Engel W, Fietzek H, Hermann M, Kolarik V (2000) J Phys IV 10:497
Norby T (1993) J Phys IV 3:99–106
Henry S, Mougin J, Wouters Y, Petit JP, Galerie A (2000) Mater High Temp 17:231
Young D (2008) High temperature oxidation and corrosion of metals. Elsevier Corrosion Series, Series Editor: Tim Burstein, p 490
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buscail, H., Rolland, R., Issartel, C. et al. Effects of water vapour on the oxidation of a nickel-base 625 alloy between 900 and 1,100 °C. J Mater Sci 46, 5903–5915 (2011). https://doi.org/10.1007/s10853-011-5544-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-5544-2