Abstract
Aluminium casting alloys containing Mg and Cu in addition to Si were investigated with respect to their potential to be foamed. The kinetics of foam expansion of different alloys was studied and the resulting structures were characterised. Of the stages of evolution of foams, namely (i) pore nucleation, (ii) foam growth in the semisolid state, (iii) further expansion in the fully liquid state, the latter two were explored. Expansion in the semisolid state could be related to the available liquid fraction. Mg-containing Al–Si alloys yielded a less coarse and more uniform pore structure than the other alloys investigated. However, achieving a high volume expansion required restriction to a narrow process window and led to the suggestion of AlMg4Si8 as a practical alloy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When metallic foams are made by expanding densified mixtures of metal powders and blowing agents (‘PM process’), often few very large pores are observed [1]. Such pores act as weak points of the structure and give rise to a large scatter of the mechanical properties. The premature gas release due to the mismatch between the decomposition temperature of the blowing agent (for aluminium alloys mostly TiH2) and the melting temperature of the alloy is thought to be a major reason for the inferior cell structure [1–4]. One strategy to solve this problem is to delay hydrogen release from TiH2, i.e. to shift it to higher temperatures. Heat treatments leading to partial decomposition and/or pre-oxidation of the powder surface [5, 6] or surface coatings [4, 7] have been applied. A complementary strategy is to change the alloy composition to obtain lower melting temperatures. Although this strategy appears promising, research in this field has not been very systematic. An additional motivation for the usage of alloys with a low melting temperature range is the production of ‘aluminium foam sandwhich’ (AFS), a commercially used metal foam product [8, 9]. Foaming of AFS requires melting and foaming of a core layer at low temperatures at which the material of the face sheets remains solid. As usage of high-strength but low-melting face sheet alloys is desirable for good overall mechanical performance [10], foamability of the core at low temperature is essential [11].
Al–Si alloys are well established in metal foaming. The casting alloy AlSi7 has frequently been used in the past [12, 13] and Si contents up to 12% have been reported [14]. The first PM metal foam part produced in series is made of AlSi11 [15]. A systematic study of foam expansion in the Al–Si system showed an optimum at Si contents of 6–7 wt% Si [16], which corresponds to ≈50% eutectic melt at the eutectic temperature of 577 °C. However, binary Al–Si alloys do not lead to satisfactory pore structures, which is why further alloying additions were proposed. Alloying elements that lower the melting point include Mg and Cu. Al–Si–Cu alloys have been applied in metal foaming for more than 10 years [17], mainly as foam core material for AFS sandwich panels [9, 18]. Al–Mg–Si with low Mg and Si contents (6000 series) were studied since they can be age-hardened [19]. Further addition of up to 1 wt% Mg to Al–Si alloys turned out to decrease the achievable volume expansion, which was ascribed to the increased oxidation rate [20]. Surprisingly, the usage of even higher Mg amounts was found to lead to promising results in the run-up to this study [21]. At present, with the development described in this work, there is a chance to replace Al–Si–Cu alloys by Al–Mg–Si casting alloys [22, 23] and in this way to reduce weight, corrosion problems and the risk of embrittlement. Zn could also be used as an addition, but due to its high solubility in Al very high contents would be required for an effective shift of the melting range. Lehmhus and Busse [2] studied quarternary Al–Si–Cu–Zn alloys, but did not find significant improvements through Zn addition.
The first aim of this study is to quantitatively evaluate the potential of some new Mg-containing alloys that promise improved pore structure and higher maximum expansion. In a second step, reasons for the beneficial effect of the high Mg addition are sought. For this, the expansion behaviour as well as the pore structure development has been studied for a number of alloys.
Methods and materials
Precursor preparation
Figure 1 gives a schematic overview of the alloy systems investigated in more detail after a wider alloy screening had been carried out. Eight different alloys belonging to four alloy systems were selected. AlSi6 is the starting point, being similar to the casting alloy A357 that is sometimes used in metal foam technology [24]. This alloy is modified by adding Cu and/or Mg in the next step.
Strategy of alloy optimisation based on adding Mg, Cu or Si to the starting alloy AlSi6. Solidus (TS) and liquidus (TL) temperatures of the individual alloys are calculated values [27]. The diagonal arrow for Si points into the third dimension
The Al–Si–Cu alloys studied are variations of alloy A319 that is in use already as a base alloy for foams [9, 18]. The rationale is to increase the amount of melt present at the ternary eutectic temperature (525 °C) by adding copper.
Al–Mg–Si alloys with high Mg contents have not yet been considered suitable alloy candidates in the literature, where just foams based on wrought alloys of the 6000 series have been described [13, 19]. The main feature of these Mg-rich alloys is the high amount of melt available at the eutectic temperature (558 °C): It increases from 18 wt% for AlMg6Si6 to 48 wt% for AlMg4Si8, see Fig. 2.
Liquid fractions as a function of temperature for different alloys. Calculations were carried out using the software JMatPro [27]
By using a quarternary alloy, the solidus temperature can be further decreased. To evaluate the potential of this strategy, alloy AlMg4Si6Cu4 was also included in this study.
The different alloy compositions were realised by mixing elemental powders or master alloy powders as specified in Table 1 in a tumbling mixer for 15 min. In addition, the mixture contained 1 wt% TiH2 powder that had been heat-treated in air for 3 h at 480 °C to delay hydrogen evolution during foaming. Such pre-treated powder starts releasing hydrogen above about 520 °C at heating rates of 0.33 K s−1 [5], possibly at slightly higher temperatures at the faster heating rates employed in this work. Cylindrical precursor material tablets, 36 mm in diameter, 7 mm high and weighing 20 g, were produced by uniaxial pressing applying 300 MPa compaction pressure first at 20 °C for a few seconds and then at 400 °C compaction temperature for 300 s.
Measurement of foam expansion
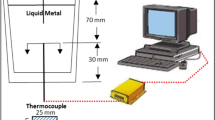
Foam expansion was measured in a special dilatometer (so-called ‘expandometer’) that was designed for in-situ metal foam expansion measurements. The instrument used was originally built at the Institute of Materials and Machine Mechanics of the Slovak Academy of Sciences, but was then modified to allow for a more precise sample temperature control and monitoring [21]. It consists of an infrared furnace and a cylindrical glass mould open only at the top in which the tablet is foamed. The mould accommodates a movable piston connected to a position sensor used to monitor foam expansion. The foaming atmosphere was air. The temperature program comprised first a heating phase applying a given constant heating power, resulting either in 2.6 K s−1 or 1.2 K s−1 heating rate (determined at 500 °C) up to a final temperature of 630 °C and then a stage of constant temperature for 300 s to allow for foam expansion. The higher heating rate represents the upper limit that can be achieved with the expandometer setup. The lower value is typical for practical foaming. Much lower values have a negative impact on foaming [25] but are irrelevant in the context of the present work. The thermocouple used for measuring the foam temperature protruded into the sample through the sample support from below.
The measured data was either displayed as volume expansion versus time or converted into expansion versus melt fraction by using the known sample temperature at any instant and applying the commercial software JMatPro that allows for calculating phase diagrams [26, 27] using the CALPHAD approach, a Gibbs energy minimising method based on experimental data bases [28]. Figure 2 shows data for the melt fraction of various alloys as a function of temperature as calculated by this approach.
For alloys that isothermally melt at the eutectic temperature, the eutectic melt fraction xE(t) at a time t between the beginning and the end of isothermal melting (t1 and t2) was linearly interpolated using the maximum possible amount of eutectic melt xE(t2) and assuming a constant heat flux \(\dot{Q}\) into the sample, i.e. neglecting possible changes of the heat transfer conditions due to sample expansion:
Since data for the density of the liquid fraction in the semisolid state is not available for the alloys used, the exact volume fraction of the melt is not known. However, by using weight fractions instead, just a small error is caused for the system Al–Mg–Si.
We determined two expansion rates, one in the range between the particular liquidus temperature and the final temperature (630 °C) and one during the following isothermal stage at 630 °C. After the experiment, cooling of the sample was initiated by turning off the furnace. The initial cooling rate was typically 1 K s−1.
Structure characterization
In-situ X-ray radiography was performed using a microfocus X-ray tube as described in Ref. [25]. The samples were 20 mm wide, 7 mm high and 5 mm thick, with the compaction (and expansion) direction arranged vertically and perpendicular to the beam direction.
Foam structure was characterised by analysing polished sections of slightly expanded foams of the different alloys using a Zeiss Axiophot 2 microscope. Pore counting was done using the free image analysis software ImageTool [29]. The minimum pore cross section that could be analysed was 450 μm2. Due to the constant analysed area of 2.8 mm × 2.2 mm it was necessary to correct for the different sample porosities P to obtain a number of pores NP related to the same initial cross section of the precursor before foaming. Therefore, NP was determined from NP,measured that represents the actual counted number by using
Highly expanded foam samples from the expandometry measurements were sectioned parallel to the expansion (compaction) axes and the foam structure was scanned using a document scanner after filling the pores with a contrast enhancer [30]. Pore size distributions were again obtained using ImageTool. The minimum cross section required for safe pore identification was 1 mm2 in this case.
Results
Foam expansion
Figure 3 displays the temperature and volume expansion course of the alloy AlMg4Si6 for both heating rates applied in this study. The solidus and liquidus temperatures, TS and TL, are clearly correlated to kinks in the temperature course (‘A’ and ‘B’). Below the solidus temperature, no foam expansion is observed and just the thermal expansion of both the sample and instrument gives rise to a slight linear increase. After TS has been reached, foam expansion sets in. For all the alloys investigated, the expansion rates depend on the heating rate. Shortly before reaching the final temperature of 630 °C, the expansion rate for the high heating rate (slope 1 in Fig. 3) largely exceeds the expansion rate at the low heating rate (slope 3). After being at the final temperature, the expansion rates immediately drop to lower values (slopes 2 and 4 in Fig. 3).
Temperature and expansion curves for alloy AlMg4Si6 foamed at two different heating rates. Broken lines labelled (1) to (4) mark expansion rates in different foaming stages. The solidus and liquidus temperatures TS and TL are marked by horizontal lines, the heat effects observed at these temperatures are marked by letters ‘A’ and ‘B’
In Fig. 4, the expansion is shown as a function of temperature. Here, expansion is nearly independent of the heating rate. The calculated melt fraction as a function of the temperature and the two expansion curves show a clear similarity, which suggests to compare volume expansion as a function of melt fraction for various alloys, see Fig. 5. In this representation, foam volume increases monotonically with the melt fraction for all the alloys. Only the two expansion curves of the Cu-containing alloys show a slightly delayed expansion compared to the other alloys in the region up to 50% melt fraction. The degree of expansion after reaching TL is very similar for all the alloys except for AlMg6Si6 where expansion at melt fractions >50% has largely slowed down.
Expansion curves for AlMg4Si6 as taken from Fig. 3 but displayed as a function of temperature up to T = 630 °C. Triangles melt fraction calculated with the software JMatPro. ‘C’ marks artefacts caused by varying heat contact conditions
All the maximum foam expansions are compared in Fig. 6. The highest expansion values were assumed by alloys AlSi6 and AlMg4Si8. The values achieved by the Al–Si–Cu alloys are just slightly lower for the high and on the same level for the low heating rate. Within the Al–Si–Cu alloy series there is no significant trend as a function of Cu content. The Al–Si–Cu alloys show the same maximum expansion for the two heating rates applied, while all the other alloys expand slightly more at high heating rates. Unlike Al–Si–Cu, the Al–Mg–Si alloy system exhibits a trend of higher maximum expansion with a higher amount of eutectic melt produced isothermally during foaming. The decrease of this eutectic melt fraction from 48% for AlMg4Si8 to 18% for AlMg6Si6 is associated with a decrease of maximum expansion of ≈30%. The quarternary alloy AlMg4Si6Cu4 and alloy AlMg6Si6 show the lowest expansions of all the alloys studied.
The expansion rates of the different alloys in the range between the liquidus temperature and the final temperature (630 °C) are compared in Fig. 7a. These values correspond to slopes 1 and 3 in Fig. 3. Experiments made with high heating rate produce a significantly higher expansion rate than the ones with low rate. No clear dependence on the alloy composition or the melting temperature range is visible.
The expansion rates determined during expansion at constant temperature (630 °C) are given in Fig. 7b (slopes 2 and 4 in Fig. 3). Here, the heating rate has no obvious influence, i.e. after being at the final temperature, the rate at which this temperature was reached played no role any more. The expansion rates in this stage are low for all the alloys, ranging from 0.015 mm s−1 to 0.055 mm s−1, and for alloy AlMg4Si6Cu4 the expansion rate is close to zero. As seen from Fig. 7, for all the alloys, the expansion rates decrease sharply after the isothermal stage has been reached. This change is more pronounced for the higher heating rate.
Development of pore structure
For one of the alloys, Fig. 8a shows the pore structure in early foaming stages. The first step of evolution is the nucleation of many small individual pores that then start to grow. Most of them are nearly equiaxed and have a preferred orientation that can be determined using quantitative analysis. The pores often arrange into linear arrays, see (2), that more frequently appear to be oriented perpendicular to the compaction axis, although other orientations can also occur. The pores in these arrays merge to larger elongated pores in a later stage, see (1). In Fig. 8b, a closeup shows such an array in a different alloy, see (3). Here, the material between the pores of the array had been partially liquid, while mainly pore-free areas in the direct vicinity of the pore array show no sign of prior melting.
a Typical early stage pore structure (P2D = 31.7%, alloy AlMg4Si6) showing the characteristic arrays of pores (2) and larger pores with irregular shape that emerged from those arrays (1). b Close-up of transition regime between individual pore arrays (3) and larger pores surrounded by melt-rich zone in an earlier stage (3) (P2D = 7.8%, alloy AlSi6Cu4). Compaction direction is from top to bottom in both cases
The number of detected pores related to the original precursor volume is given in Fig. 9. A maximum value of the number of such pores is observed for 2D-porosities of 20–25%. Mg-containing alloys have significantly more pores in the region of 10–25% 2D-porosity than Mg-free ones.
X-ray images of the foams in an early stage after pore nucleation as shown in Fig. 10 reveal cracks that propagate horizontally, i.e. perpendicular to the compaction direction for 30% expansion. The pores in the AlMg4Si6 alloy appear less cracklike than in AlSi6Cu4 or even AlSi6. In the latter case of a AlSi6 alloy, visible cracks lengths can reach ≈1 cm (50% of the sample width). The crack-like structure of the early-stage pores results in an anisotropic expansion after they get inflated by the blowing gas since they then expand perpendicular to their orientation, i.e. parallel to the compaction direction. The particular alloys show different characteristics of anisotropy of expansion even in a later stage (area expansion 100%, Fig. 10b), where the stronger anisotropy of expansion compared to the other alloys leads to a nearly rectangular contour of the AlSi6 sample. AlSi6Cu4 shows a rounder shape due to less anisotropic expansion, while the AlMg4Si6 foam is the roundest.
Statistical averages of macroscopic pore cross sections in fully expanded foams including pores exceeding 1 mm2 area are given in Fig. 11. The Pn-values specified indicate the cell area fraction of n% of the pores (by number) does not exceed (P50 is then the median value). The span between P95 and the maximum values of the distributions is a measure for the homogeneity of the pore structure since it reflects the presence or absence of very large individual pores. Such differences are especially small for the alloy AlMg4Si8. The absolute values of the other parameters indicate whether the structure formed by the majority of the pores is coarse or fine. One can identify the following ranking for the coarseness of the structure:

The biggest difference is found between the group of the Mg-free (4 left in Fig. 11) and Mg-containing alloys (4 right in Fig. 11).
Discussion
It was found that by using Mg as an alloying element, less coarse and more homogeneous Al–Si-based foams can be manufactured (Fig. 11). However, Mg can also have an adverse effect on expansion if used in the wrong proportion to other elements, as the expansion measurements showed (Fig. 6). Therefore, a trade-off between beneficial and adverse effects of Mg has to be considered. These effects shall be discussed in the following. The alloy AlMg4Si8 represents a good compromise due to the good expansion and fine pore structure found (Figs. 6, 11) and Fig. 12, which is why this alloy has been further studied [22, 23].
Figure 5 shows that during melting of the alloy foam expansion is rather correlated with the melt fraction than with the temperature. Different alloys reach the same melt fraction at different temperatures, see Fig. 2, which is why diagrams such as Fig. 4 show initial foam expansion at different temperatures for different alloys. In contrast, in Fig. 5, the different alloys show a similar course with only few deviations: The delay for the Cu-containing alloys between 30% and 50% melt fraction could be an artefact, as in the powder compacts containing Cu powder local melting around individual powder particles produces a lot of eutectic melt at lower temperatures, but this melt is contained in local pockets at the location of former copper particles [31]. Oxide layers around the former powder particles could hamper the equalisation of concentration gradients. The actual global melt fraction can therefore be lower than the value determined from the equilibrium phase diagram and used in Fig. 5. A correction for this would move the curves in the direction of the arrow given in Fig. 5, but this correction cannot be quantified. As Mg is already dissolved in the AlMg50 master alloy used, no such local melting is observed in the Al–Mg–Si alloys. Since Mg also helps breaking the oxide films during powder pressing, Mg powder compacts melt more homogeneously.
The decomposition rate of the blowing agent strongly depends on temperature [5, 32–34]. Therefore, the correlation found between melt fraction and expansion—even though not perfect—is not trivial. The volume gain of the foam in the semisolid melting range is limited by the extent to which the matrix can be stretched and accommodate gas bubbles, and this ability increases with the liquid fraction. Gas supply at this stage is necessary but not a controlling factor. Only after complete melting, further foaming will be largely controlled by the strongly temperature-dependent gas release from the blowing agent.
An important aspect in this foaming stage is the development of the pore structure. Expanded foams in the liquid can be very stable [35, 36] unless the temperature exceeds a critical value [37]. But even if the temperature is controlled properly, coalescence can always be observed in expanding foams, clearly visible in in-situ X-ray images as rupture of films separating adjacent bubbles [11, 25, 37, 38]. In this work, we show that coalescence takes place already in very early stages. The structure of just slightly expanded samples as displayed in Fig. 8 shows that pore coalescence already occurs on the microscopic scale below 30% porosity. X-ray radioscopy cannot reveal such processes in the still rather dense structure. Figure 9 shows that for large porosities the number of pores exceeding an area of 450 μm2 is still increasing, which means that smaller pores are inflated to such an extent that they are counted (the actual nucleation process takes place at much smaller length scales and is not reflected by our image analysis). The peak in pore number observed in Fig. 9 indicates that after a certain level of porosity has been reached (≈25%), the coalescence rate exceeds the rate at which countable pores are being generated and the total number of pores decreases. In two-dimensional images, coalescence appears as the merger of linear pore arrays. Due to the symmetry with respect to the compaction axis there is good reason to assume that these observed arrays are in reality planar structures in three-dimension [39].
While the merger of small pores at this stage exhibits no clear preferential direction, X-ray analysis of the same materials in later stages, see Fig. 10, shows oriented crack-like structures as a result of anisotropic processes, in agreement with previous findings [38–40]. Pore growth that resembles crack propagation as observed in the semisolid state is often ascribed to the temperature mismatch between TiH2-decomposition and melting [1–4]. This leads to a build-up of gas pressure already in the solid or semisolid state in which the metal cannot form expanding bubbles. In this stage, foam expansion is anisotropic.
Compared to AlSi6, both Cu and Mg additions reduce the formation of cracklike structures, but the addition of magnesium has a stronger influence than that of copper, see Fig. 10. At first sight, this appears counter-intuitive, as the solidus temperature is shifted to a lower level by Cu (525 °C) than by Mg (558 °C), i.e. melt is provided at the lowest temperatures in Cu-containing alloys. However, not only the temperature but also the amount of melt that is produced after exceeding the ternary eutectic temperature matters and phase diagram analysis indeed shows that the melt fraction achieved at the solidus temperature of Al–Mg–Si alloys (558 °C) is much higher than that of the Al–Si–Cu alloys (525 °C) at the analogous temperature, see Fig. 2.
Knowing this, the model illustrated schematically in Fig. 13 is proposed. Based on the microstructural investigations we assume that in the melting range islands of still solid material are bridged by both solid and liquid material of smaller cross section. During further heating, the latter grew at the expense of the former. Caused by the increasing pressure provided by the blowing agent, two different types of pore coalescence are possible: rupture of solid bridges that occurs preferentially in the pressing direction and rupture of liquid films that occurs isotropically. The reason for the lower strength of the still solid material in the vertical direction is that this is also the pressing direction of the powder. Strength-reducing oxide films are thought to remain unchanged when faying surfaces are pressed against each other, while the oxides are fragmented when powder particles slide against each other. This gives rise to a lower strength in the pressing direction.
In Al–Si–Cu alloys, melt is formed at 525 °C, but only in small amounts. This weakens the structure considerably. The small amount of eutectic melt, see Fig. 2, is first confined to small pockets and later spreads along the grain boundaries [31]. It cannot bridge the solid constituents that are pushed apart by the gas which in turn gives rise to crack formation. This appears as an analogue to hot-tearing in many Al alloys containing low amounts of eutectic melt, although that effect occurs during solidification [41]. In Al–Mg–Si alloys, the matrix remains its solid integrity up to 558 °C. The integrity of the matrix is further strengthened by the presence of Mg since it helps breaking the oxide films covering the individual powder particles during hot pressing and sintering [42]. At 558 °C, a large amount of eutectic melt is generated isothermally and liquid films dominate quickly. Other than solid bridges, liquid films are stretchable and are able to stop crack propagation and prevent coarsening of the structure in the semisolid state.
In addition to their effect on the melting temperature, alloying elements, first of all Mg, modify melt properties such as viscosity, surface tension and affinity to oxidation. Surface tension is a potential driving force for cell wall rupture. All alloying elements used reduce the surface tension of Al [43], but the influence of Mg is much higher than the effect of other elements. Mg could therefore be beneficial for film stabilisation for this reason, especially as it enriches at the surface [44].
Oxide skins or particles at the metal/gas interface play a crucial role for metal foam stability [16, 35, 45–47]. During expansion, oxide skins repeatedly rupture, but new oxides form, preferably at the thinnest point of the oxide skin [41]. Oxygen is present in the precursor as air entrapped during compaction or as adsorbate on the powder surfaces (mainly H2O and hydroxides) [48, 49]. In both cases, Al and Mg can react with oxygen [50]. Another influence of oxidation (beside that on film stability) is the mechanical resistance of the oxidised inner surfaces against expansion [47]. The smaller the pores, the higher the inner surface and hence the resistance. The quaternary alloy with the smallest pores, see Fig. 11, and the alloy containing 6 wt% Mg both have a poor maximum expansion, see Fig. 6. The quaternary alloy even shows a complete loss of expansion rate after full melting, see Fig. 7b. This underlines that a high Mg content in conjunction with small pores restricts foam expansion. On the other side, the coarse structure of AlSi6 foam explains the high expansion reached (Fig. 6).
Melt viscosity is an often discussed possible influence factor for cell wall stability, although recent microgravity research showed no significant gain in stability in the absence of drainage [36]. Unfortunately, the viscosity of the liquid in the films of a semisolid foam can hardly be quantified. Known is just the ability of Mg-containing alloys to form finely dispersed stable solid Mg2Si particles in the relevant temperature region, which could lead to an increased apparent viscosity of the melt [51]. Such Mg2Si particles have actually been found in solidified samples [21].
The present paper mainly deals with foam expansion in the melting range of the base alloy, but the stages before and after are equally important. The entire foaming process can be subdivided into:
-
the nucleation stage in which the initial pores are created. Nucleation is observed only indirectly in the present work,
-
the melting stage in which the fraction of already melted material mainly determines foam expansion. In this stage, the foundation has to be layed for a uniform pore structure and a further smooth and high expansion,
-
the expansion stage of the fully liquid alloy, which is strongly influenced by the charactersistics of TiH2 decomposition. As soon as the temperature increase comes to an end, foam expansion slows down or even stops since further gas evolution can only be triggered by increasing temperatures.
At present, a thorough investigation of the nucleation stage is lacking in the literature. Only few studies have been carried out, e.g. using ultra-small scattering techniques or electron microscopy [52]. Gathering further information should be the subject of further work.
Conclusions
-
Mg and Cu additions to Al–Si alloys can improve both the pore structure and the maximum expansion.
-
Mg shows clear advantages over Cu as third alloying element.
-
The alloy AlMg4Si8 represents a good trade-off between the beneficial effects of Mg and adverse factors.
-
The beneficial action of Mg is to provide a high melt fraction at the solidus temperature and possibly also to decrease both surface tension and viscosity. Moreover, Mg improves sintering during powder pressing.
-
The proneness of Mg to oxidation could help to stabilise the foams but can also hinder foam expansion.
-
Foam expansion in the semisolid range is directly correlated to melt fraction.
-
Pore coalescence was found to start very early, i.e. at <10% pore fraction, and to reduce the number of pores above 25% pore fraction.
References
Matijasevic B, Banhart J (2006) Scripta Mater 54:503
Lehmhus D, Busse M (2004) Adv Eng Mater 6:391
Ibrahim A, Körner C, Singer RF (2008) Adv Eng Mater 10:845
Proa-Flores PM, Drew RAL (2008) Adv Eng Mater 10:830
Matijasevic B, Banhart J, Fiechter S, Görke O, Wanderka N (2006) Acta Mater 54:1887
Lehmhus D, Rausch G (2004) Adv Eng Mater 6:313
Yang Z, Fang J, Ding B (2005) J Colloid Interface Sci 290:305
Seeliger H-W (2002) Adv Eng Mater 4:753
pohltec metalfoam GmbH. Homepage of pohltec metalfoam GmbH (Kln, Germany). https://doi.org/www.metal-foam.de
Lehmhus D, Busse M, Chen Y, Bomas H, Zoch H-W (2008) Adv Eng Mater 10:863
Banhart J, Stanzick H, Helfen L, Baumbach T, Nijhof K (2001) Adv Eng Mater 3:407
Brinkers W, Banhart J (1999) J Mater Sci Lett 18:617
Duarte I, Banhart J (2000) Acta Mater 48:2349
Banhart J, Weber M, Baumeister J (1996) Mater Sci Eng A 205:221
Alulight International GmbH. Homepage of Alulight International GmbH (Ranshofen, Austria). https://doi.org/www.alulight.com
Weigand P (1999) PhD thesis, RWTH Aachen, Aachen, Germany
Banhart J, Baumeister J (1998) J Mater Sci 33:1431. doi:https://doi.org/10.1023/A:1004383222228
Shabestari SG, Wanderka N, Seeliger W, Banhart J (2006) Mater Sci Forum 519–521:1221
Lehmhus D, Banhart J, Rodriguez-Perez MA (2002) Mater Sci Technol 18:474
Simancik F, Behulova K, Bors L (2001) In: Banhart J, Ashby MF, Fleck NA (eds) Cellular metals and metal foaming technology, p 89
Helwig H-M (2009) PhD thesis, Technische Universität Berlin, Berlin
Helwig H-M, Banhart J, Seeliger H-W (2008) German patent application 10 2008 027 798.3, patent pending
Helwig H-M, Hiller S, Garcia-Moreno F, Banhart J (2009) Metall Mater Trans B 40:755
Baumgärtner F, Duarte I, Banhart J (2000) Adv Eng Mater 2:168
Garcia-Moreno F, Fromme M, Banhart J (2004) Adv Eng Mater 6:416
Saunders N (2004) In: Nieh JF, Morton AJ, Muddle BC (eds) Proceedings of 9th international conference on aluminium alloys (ICAA9), p 96
Sente Software Ltd. Practical software for materials properties JMatPro. https://doi.org/www.sentesoftware.co.uk
Kattner UR (1997) J Met 49:14
University of Texas Health Science Center. Homepage of UTHSCSA ImageTool. https://doi.org/ddsdx.uthscsa.edu/dig/itdesc.html
Wübben T (2003) PhD thesis, Universität Bremen, Bremen
Rack A, Helwig H-M, Rueda A, Matijasevic-Lux B, Banhart J, Helfen L, Baumbach T, Goebbels J (2009) Acta Mater 57:4809
von Zeppelin F, Hirscher M, Stanzick H, Banhart J (2003) Compos Sci Technol 63:2293
Kennedy AR, Lopez VH (2003) Mater Sci Eng A 357:258
Bhosle V, Baburaj EG, Miranova M, Salama K (2003) Mater Sci Eng A 356:190
Körner C, Arnold M, Singer F (2005) Mater Sci Eng A 396:28
Garcia-Moreno F, Jimenez C, Mukherjee M, Holm P, Weise J, Banhart J (2009) Colloids Surf A 344:101
Stanzick H, Wichmann M, Weise J, Helfen L, Baumbach T, Banhart J (2002) Adv Eng Mater 4:814
Banhart J, Stanzick H, Helfen L, Baumbach T (2001) Appl Phys Lett 78:1152
Rodriguez-Perez MA, Solorzano E, de Saja JA, Garcia-Moreno F (2008) In: Lefebvre L-P, Banhart J, Dunand D (eds) Porous metals and metal foaming technology. DEStech Publishing, Pennsylvania, p 75
Helfen L, Baumbach T, Stanzick H, Banhart J, Elmoutaouakkil A, Cloetens P (2002) Adv Eng Mater 4:808
Campbell J (2003) Castings, 2nd edn. Butterwoth-Heinemann, Oxford, UK
Shirzadi AA, Assadi H, Wallach ER (2001) Surf Interface Anal 31:609
Park S-H, Hur B-Y, Kim S-Y, Ahn D-K, Ha D-I (2002) In: Hong C-P, Kim D-H, Kim K-Y (eds) Proceedings of the 65th World Foundry Congress Gyeongju, Korea, p 515
Awe OE, Akinlade O, Hussain LA (2006) Surf Sci 600:2122
Kaptay G (2004) Colloids Surf A 230:67
Wübben T, Odenbach S (2005) Colloids Surf A 266:207
Dudka A, García-Moreno F, Wanderka N, Banhart J (2008) Acta Mater 56:3990
Garcia-Moreno F, Banhart J (2007) Colloids Surf A 309:264
Jimenez C, Garcia-Moreno F, Banhart J, Zehl G (2008) In: Lefebvre LP, Banhart J, Dunand D (eds) Porous metals and metal foaming technology. DEStech Publishing, Pennsylvania, p 59
Birks N, Meier GH (1983) Introduction to high temperature oxidation of metals. Edward Arnold Ltd, London
Paes M, Zoqui EJ (2005) Mater Sci Eng A 406:63
Banhart J, Bellmann D, Clemens H (2001) Acta Mater 49:3409
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Helwig, HM., Garcia-Moreno, F. & Banhart, J. A study of Mg and Cu additions on the foaming behaviour of Al–Si alloys. J Mater Sci 46, 5227–5236 (2011). https://doi.org/10.1007/s10853-011-5460-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-5460-5