Abstract
A solvothermal method is described for preparing nanomaterials of titanium dioxide (TiO2) while controlling the growth of rod-shaped nanomaterials. Titanium (IV) isopropoxide (TTIP) and butyl ether were chosen as the precursor and solvent, respectively. Oleic acid and decanoic acid were used as the surfactant. TiO2 nanospherical particles and nano-rods were synthesized in mass quantities by adjusting the kind of surfactant, the concentration of the precursor, and the solvothermal reaction temperature. The synthesized TiO2 nanospherical particles and nanorods were uniform and transparent in the toluene. The TiO2 nanospherical particles were about 3.5 nm in size. The TiO2 nanospherical particles and nanorods were of a highly crystalline anatase structure. The direction of growth of the TiO2 nanorods was [001] and the band gap energy of the TiO2 nanorods, evaluated by optical absorption, was 3.34 eV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
TiO2, an n-type wide band gap semiconductor, has a variety of promising applications, for example in dye sensitized solar cells [1–3], photocatalysts [4, 5], lithium batteries [6–8], and photochromic devices [9, 10], due to its attractive optical, electrical, chemical, and catalytic properties. One-dimensional TiO2 materials, such as nanotubes, nanowires, nanofibers, and nanorods have attracted particular interest due to their unique microstructure and promising functions [11–14]. One-dimensional TiO2 nanocrystals exhibit a high surface to volume ratio, improved charge transport afforded by their dimensional anisotropy and an increased number of delocalized carriers [11, 15, 16]. Depending on these properties and applications, TiO2 materials must fulfill a wide variety of requirements in terms of particle size, size distribution, morphology, crystallinity, and phase.
Various methods, such as sol–gel processing [17–19], hydrothermal reactions [20–22], and wet-chemical methods [23, 24] have been applied to improve the size and shape of one-dimensional TiO2 nanomaterials. However, due to the fast reactivity of titanium alkoxides and the forming of large complex molecules upon exposure of titanium alkoxides to ambient humidity, the concentration of titanium alkoxides was so low that only a small quantity of TiO2 nanocrystals could be made by non-aggregation of TiO2 nanoparticles. Although these problems in the preparation of TiO2 nanomaterials are solved through the modification, one of the limitations of these techniques is a low crystallinity. In order to improve this factor, most of the previous researches have been performed employing long reaction time (over 20 h) and additional heat-treatments (over 600 °C).
Recently, a solvothermal process [25–37] has been widely utilized in the synthesis of TiO2 nanostructures, which makes easier to control the size, crystallinity, morphology, and surface chemistry of TiO2 compared with other synthetic processes. Since the reaction is performed at an elevated pressure, more crystallized and denser structures have been obtained at a given temperature.
Thus, in this work, we combined the solvothermal process and reverse micelle technique to obtain more crystallized and controlled morphology of titania nanoparticles without aggregation. We have chosen TTIP, decanoic acid, oleic acid, and butyl ether as a TiO2 precursor, surfactant, and solvents, respectively. The carboxylic acids of long hydrocarbon chain form a stable reverse micelle structure in non-polar ether. During the solvothermal reaction, it has been reported that the application of surface selective surfactants would be finely tune the growth rate of the faces to follow the resulting shape evolution process [14, 38–40]. In particular, carboxylic acids bind very strongly to {001} face of anatase, one of the TiO2 crystal phases. This fact suggests that we can synthesize one-dimensional TiO2 nanorods bound to carboxylic acids at a low temperature [40]. Various variables (e.g., the amount of concentration of carboxylic acids, the kind of carboxylic acids, the reaction temperature, and the reaction time) have been studied. From the results, we will describe the reaction mechanism of the TiO2 formation process.
Experimental procedure
0.015 mol of titanium (IV) tetraisopropoxide (Ti[OCH(CH3)2]4, 97%, Aldrich, TTIP) was dissolved in 100 mL of butyl ether (C4H9OC4H9, 99%, Aldrich), and the oleic acid (CH3(CH2)7CH=CH(CH2)7COOH, 90%, Aldrich) and decanoic acid (CH3(CH2)8COOH, 98%, Sigma) with a molar ratio of TTIP:acid = 1:1, 1:2, 1:3, and 1:4 were added dropwise in nitrogen atmosphere. The mixture was stirred for 1 h and then the resulting transparent yellow solution was transferred to an autoclave. For the solvothermal reaction, the temperature of the autoclave was raised to 250, 280, or 300 °C at a rate of 5 °C/min, and held for 2, 4, 6, or 8 h. The reactor pressure was about 30 atm. After the solvothermal reaction, we obtained a deep yellow TiO2 colloidal suspension. Excess ethanol was added to TiO2 colloidal suspension and the mixture was transferred to round bottom flask, tempered to 40 °C in a water bath, and held under a reduced pressure for 30 min using a vacuum pump to remove surfactant residuals. This step was repeated five times. The resulting white powder could easily be re-dispersed in a non-polar solvent. Synthesized TiO2 was then dispersed into toluene resulting in a transparent solution. The synthesized TiO2 nanorods were characterized by X-ray diffractometry (XRD, DMAX 2500, Rigaku, Japan) using Cu-Kα radiation (λ = 1.54178 Å) and a high resolution transmission electron microscope (TEM, CM200, Philips, Netherlands). Infra-red (IR) and Raman spectra were collected with an FT-IR spectrometer (Presitige-21, Shimadzu, Japan) and an FT-Raman spectrometer (RFS-100/S, Bruker, Germany), respectively. Optical absorption was recorded at room temperature with UV–vis spectroscopy (UV 2450, Shimadzu, Japan).
Results and discussion
Solvothermal reaction of the TiO2 nanocrystals with carboxylic acid
In this study, we have demonstrated a solvothermal process, controlling the concentration of carboxylic acids, the kind of carboxylic acids, the reaction temperature, and the reaction time. The result was a successful crystallization of TiO2 nanoparticles and nanorods. Titanium alkoxides are known to readily react with carboxylic acids by competitive pathways shown below [41]:
Poly nuclear oxo complexes have been produced, depending on experimental conditions.
Figure 1 shows the FT-IR spectra of the TiO2 suspension: (a) the mixture of TTIP and oleic acid in dibutyl ether, and (b) as-synthesized TiO2 nanorod. In Fig. 1a, two absorption bands are observed at 1,710 and 1,740 cm−1 resulting from the carbonyl group band of the free oleic acid and the ester, which was produced from the mixing of the materials [33, 42, 43]. After the reaction proceeds for 6 h, the absorption peak resulting from the carbonyl group band of oleic acid at 1,710 cm−1 almost disappeared. On the other hand, the intensity of the carbonyl band originating from the produced ester at 1,740 cm−1 increased sharply as shown in Fig. 1b. This indicates that ester was produced from the carboxyl group of the oleic acid and alkyl group of the TTIP in accordance with Eq. 1b. The medium intensity band at 1,600 cm−1 and the very broad band around 3,000–3,300 cm−1 in Fig. 1a result from the bending vibration and the stretching vibration of the Ti–OH bonds [14].
From the FT-IR analysis, we conclude that these hydroxide groups in TiOH forms the TiO2 nanocrystals by the condensation reaction at elevated temperature and pressure. We believe that complete disappearance of these peaks from the hydroxide groups in TiOH as shown in Fig. 1b supports above-mentioned mechanism, Eq. 1b.
The crystalline phase, size, and morphology of the TiO2 nanocrystals
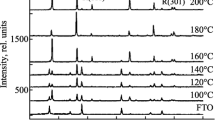
As indicated by the XRD patterns in Fig. 2, the TiO2 particles prepared at 250 °C were amorphous at 0.015 mol oleic acid. On the other hand, the TiO2 nanoparticles prepared at 300 °C were pure anatase phase with lattice constants of a = b = 3.785 Å and c = 9.514 Å (JCPDS card No.: 21-1272), which is in agreement with the result obtained from FT-Raman (Fig. 3). Moreover, when the concentration of the oleic acid was increased, the tendency of the TiO2 nanocrystals with regard to reaction temperature was the same, which denotes that the amount of the surfactant is not a parameter that controls the crystalline phase of TiO2. The average crystallite sizes of TiO2 nanoparticles at 300 °C were calculated from the full-width at half-maximum intensity of the (101) peak at 2θ = 25.28° using the Scherrer equation. The average crystallite size of the TiO2 nanoparticles was about 3.5 nm, which was very small as compared with those obtained by other methods. On the other hand, the average crystallite size of the TiO2 nanoparticles prepared by decanoic acid was 5.5 nm. This indicated that the chain length of the carboxylic acids is a very important parameter controlling the particle size. This feature is also observed by TEM study.
Figure 4 shows low magnification TEM images of synthesized nanostructures. The concentration of the carboxylic acids greatly influences the morphology of the final product. When the content of decanoic acid was 0.015 mol, synthesized TiO2 nanoparticles were aggregated. However, increasing the content of decanoic acid decreased the aggregation of TiO2 nanoparticles and increased their dispersibility. Moreover, when the amount of the decanoic acid was 0.045 mol, the synthesized TiO2 was rod-shaped. This feature also appeared in the TiO2 nanocrystals prepared with the oleic acid. However, when the TiO2 samples were synthesized with different amounts of oleic acid, dispersibility improved and the rod shape and anatase phase of the TiO2 were distinct compared with that synthesized with decanoic acid. These results indicate that oleic acid is useful for preparing TiO2 of uniform rod shape. In Table 1, we have summarized these tendencies of the morphology of TiO2 nanocrystals synthesized with different carboxylic acids.
When the content of the oleic acid was 0.06 mol, the average size of the rod-shaped TiO2 was about 3.5 × 30 nm (Fig. 5a). Figure 5b is an HRTEM image of TiO2 nanorods synthesized with 0.06 mol of oleic acid. These nanorods are structurally uniform with an interplanar space of about 3.5 Å, which corresponds to the distance between two (101) planes of anatase phase TiO2. In general, the reduction in surface energy is the primary driving force for simple particle growth, and morphology evolution is driven by the further reaction in energy due to minimization of the area of high surface energy faces. According to the Donnay–Harger rules, the surface free energy of the {001} faces is nearly 1.4 times larger than that of the {101} faces [15, 22, 40, 44–47]. Therefore, these features determine the intrinsic instability of the nucleus; the shape evolution in TiO2 anatase can be realized by modifying the surface free energy and growth rate of the nucleus with the surface adhesion of the ligands.
Ethanol has been introduced to remove the surfactant residuals. We found out, however, that successive washing of synthesized TiO2 with ethanol brings about the change in the shape of nanorods as depicted in Fig. 6. It has been observed that the length of the TiO2 nanocrystals increased with the number of washings by ethanol. We do not fully understand why the washing away of surfactant residuals causes this very interesting effect. Further studies are needed to explain this result.
The bandgap energy of the TiO2 nanocrystals
The optical band gaps of the TiO2 nanorods were studied by the UV–vis spectroscope absorbance spectra displayed in Fig. 7. The absorption coefficient (α) and the bandgap (E g) are related through the equation [48].
where v is the frequency, h is Planck’s constant, and s = 0.5 for indirect bandgap material. The Tauc plot is (αhv)1/2 vs. hv. As absorbance (A) is proportional to the absorption coefficient (α) [20], the Tauc plot, (αhv)1/2 vs. hv was replaced by (Ahv)1/2 vs. hv.
From this spectrum, the optical bandgap, obtained by dropping a line from the slope of the curve, is 3.34 eV. The reported bandgap value of the bulk anatase phase is 3.2 eV. The peak is 40 nm blue shifted from that of bulk anatase. This 40 nm blue shift seems to be the result of quantum size effects.
Conclusions
The crystal phase, size, and shape controlled TiO2 nanorods were synthesized through a solvothermal process. On the basis of the experimental results, a possible reaction mechanism of formation of TiO2 nanorods has been proposed. This process is very useful and holds several significant advantages for the synthesis of one-dimensional TiO2 nanocrystals. The prepared TiO2 nanorods were clear anatase phase. The shape and length of the TiO2 nanocrystals could be controlled by selecting the concentration and kind of surfactants and with the washing-evaporation procedure. In our future research, we will apply the TiO2 nanorods to electrodes of dye sensitized solar cells.
References
Park N, Kim KM, Kang MG, Ryu KS, Chang SH, Shin Y (2005) Adv Mater 17:2349
Yoon J, Jang S, Vittal R, Lee J, Kim K (2006) J Photochem Photobiol A Chem 180:184
Han H, Zan L, Zhong J, Zhao X (2005) J Mater Sci 40:4921. doi:10.1007/s10853-005-3880-9
Kim E, Whang C, Lee W, Kim Y (2006) J Electroceram 17:899
Kim YK, Kim EY, Whang CM, Kim YH, Lee WI (2005) J Sol-Gel Sci Technol 33:87
Exnar I, Kavan L, Huang SY, Gratzel M (1997) J. Power Sources 68:720
Kim KM, Park NG, Ryu KS, Chang SH (2002) Polymer 43:3951
Bavykin DV, Friedrich JM, Walsh FC (2006) Adv Mater 18:2807
Nussbaumer RJ, Caseri WR, Smith P (2006) J Nanosci Nanotechnol 6:459
Xu J, Li L, Yan Y, Wang H, Wang X, Fu X, Li G (2008) J Colloid Interface Sci 318:29
Koo B, Park J, Kim Y, Choi S, Sung Y, Hyeon T (2006) J Phys Chem B 110:24318
Yao Q, Liu J, Peng Q, Wang X, Li Y (2006) Chem-Asian J 1:737
Li X, Peng Q, Yi J, Wang X, Li Y (2005) Chem-Eur J 12:2383
Joo J, Kwon SG, Yu T, Cho M, Lee J, Yoon J, Hyeon T (2005) J Phys Chem B 109:15297
Cozzoli PD, Comparelli R, Fanizza E, Curri ML, Agostiano A, Laub D (2004) J Am Chem Soc 126:3868
Cozzoli PD, Kornowski A, Weller H (2003) J Am Chem Soc 125:14539
Zhou W, Cao Q, Tang S (2006) Powder Technol 168:32
Miao L, Tanemura S, Toh S, Kaneko K, Tanemura M (2004) J Cryst Growth 264:246
Monreal-Romero HA, Martinez-Villafane A, Chacon-Nava JG, Glossman-Mitnik D, Garcia-Casillas PE, Martinez-Perez CA (2005) Nanotechnology 16:1272
Liu Z, Sun DD, Guo P, Leckie JO (2007) Chem-Eur J 13:1851
Gavrilov AI, Garshev KA, Kovnir KA, Churagulov BR, Kolen′koa YV, Tretyakova YD (2005) Russ Chem Bull 54:71
Penn RL, Banfield JF (1999) Geochim Cosmochim Acta 63:1549
Wang B, Shi Y, Xue D (2007) J Solid State Chem 180:1028
Zhong Z, Lin M, Ng V, Ng GXB, Foo Y, Gedanken A (2006) Chem Mater 18:6031
Liao DL, Liao BQ (2007) J Photochem Photobiol A Chem 187:363
Wen B, Liu C, Liu Y (2005) Chem Lett 34:396
Yin S, Komatsu M, Liu B, Li R, Wang Y, Sato T (2008) J Mater Sci 43:2240. doi:10.1007/s10853-007-2071-2
Yang X, Konishi H, Xu H, Wu M (2006) Eur J Inorg Chem 2006:2229
Wu L, Yu JC, Wang X, Zhang L, Yu J (2005) J Solid State Chem 178:321
Yang S, Gao L (2006) Mater Chem Phys 99:437
Kim CS, Moon BK, Park JH, Choi BC, Seo HJ (2003) J Cryst Growth 257:309
Liu L, Zhao Y, Liu H, Kou H-Z, Wang Y (2006) Nanotechnology 17:5046
Das K, Panda SK, Chaudhuri S (2008) J Cryst Growth 310:3792
Wahi RK, Liu Y, Falkner JC, Colvin VL (2006) J Colloid Interface Sci 302:530
Supphasrirongjaroen P, Praserthdam P, Panpranot J, Na-Ranong D, Mekasuwandumrong O (2008) Chem Eng J 138:622
Wnag Q, Wen Z, Li J (2006) Inorg Chem 45:6944
Xie R, Shang JK (2007) J Mater Sci 42:6583. doi:10.1007/s10853-007-1506-0
Cozzoli PD, Kormowski A, Weller H (2003) J Am Chem Soc 125:15439
Vijaya Kumar TV, Prabhakar S, Raju GB (2002) J Colloid Interface Sci 247:275
Jun Y, Casula MF, Sim JH, Kim SY, Cheon J, Alivisatos AP (2003) J Am Chem Soc 125:15981
Sakka S (2004) Handbook of sol-gel science and technology processing characterization and applications. Kluwer Academic Publications, London
Vioux A (1997) Chem Mater 9:2292
Hay JN, Raval HM (2001) Chem Mater 13:3396
Oliver PM, Watson GW, Kelsey ET, Parker SC (1997) J Mater Chem 7:563
Barnard AS, Zapol P (2004) J Phys Chem B 108:18435
Tanner RE, Liang Y, Altman EI (2002) Surf Sci 506:251
Zhang H, Penn RL, Hamers RJ, Banfield JF (1999) J Phys Chem B 103:4656
Mor GK, Paulose M, Grimes GA (2005) Adv Funct Mater 15:1291
Acknowledgements
We gratefully acknowledge the financial support of the Korea Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, E.Y., Choi, H. & Whang, C.M. Controlled growth of TiO2 nanorods capped with carboxylate groups by the solvothermal process. J Mater Sci 45, 3895–3900 (2010). https://doi.org/10.1007/s10853-010-4448-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4448-x