Abstract
The effect of ultrasound radiation on the size and size distribution of synthesized copper particles was investigated under various concentrations of ethylene glycol (E.G.) as a capping agent. Monodispersed copper particles were produced by the reduction of an aqueous copper (II) sulfate solution at the presence of hydrazine monohydrate. X-ray diffraction and scanning electron microscopy analysis revealed that the morphology, size, and size distribution of produced particles were influenced by the reducing agent injection rate, capping agent concentration, and sonication. Increasing the injection rate of reducing agent to an amount higher than a critical value decreases the size of copper particles and also converts the monodispersed particles to polydispersed particles. Results of using a sonifier at the reduction stage revealed that finer monodispersed copper particles can be achieved at higher injection rates related to the critical value. Increasing the concentration of E.G. as a capping agent decreases the size of copper particles, while applying ultrasound radiation along with increasing the concentration of E.G. increases the size of copper particles. Morphology of particles varies by the concentration and type of the capping agent. Higher reducing agent injection rates and the application of a sonifier at the instance of reduction result in smaller spherical particles at various capping agent concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, much attention has been paid to metal nano-particles due to their novel optical, electronic, magnetic, and chemical properties resulting from their extremely small dimensions and special surface [1–5]. Among various metal particles, copper nano-particles have attracted considerable attention because of their superior thermal and electrical conductivity. Different methods have been developed for the preparation of copper nano-particles including micro emulsion techniques [6–8], laser ablation [9], radiation methods [10], wet chemical reduction [11], thermal reduction [5], metal vapor synthesis [12], and vacuum vapor deposition [13]. The wet chemical reduction method has been widely used by many researchers because of its superior ability in controlling the size and morphology of nano-particles for large-scale production. Organic solvents such as ethylene glycol (E.G.) [14], n-heptane, n-octane, n-hexane, cyclohexane, CHCl3 [15], benzene, toluene [16] and organic capping agents such as poly(vinylpyrolidone) [14], sodium dodecyl sulfate [17], hexadecyltrimethylammonium bromide [18], octadecylamine [19], ethylene diamine [20] are the most common reactants used in wet chemical method. In the reported studies, the reaction solutions were carefully deoxygenated and the processes were performed under a rigorous protection of inert gas to prevent the oxidation. Wu and Chen independently reported the synthesis of copper nano-particles in an aqueous system under a nitrogen atmosphere [18]. Kumar et al. and Dhas et al. prepared copper nano-particles by using the sono-chemical reduction method. Their product was a mixture of copper and copper oxide nano-particles [21, 22]. In sono-chemistry the main event is the creation, growth, and collapse of a bubble that is formed in the liquid. Each collapsing bubble forms a few nucleation centers whose growth is limited by the short collapse [22]. In most of these studies, organic solvents [14–16, 23], capping agents [17–20], high temperature [24], inert atmospheres [18, 21], and a limited injection rate of the reducing agent [14] have been used to control the size, distribution, and purity of synthesized copper particles. Removing the used organic solvent and capping agent under controlled atmosphere needs a tedious procedure which is time consuming and expensive.
In the present work, ultrasound radiation has been used to produce monodispersed metallic copper particles in an aqueous solution without any copper oxide by product at ambient atmosphere. The effect of ultrasound radiation and injection rate of reducing agent on the size and size distribution of synthesized copper particles was investigated under various concentrations of E.G. as a capping agent. One of the most important advantages of ultrasound radiation is that smaller spherical monodispersed copper particles can be produced at high reducing agent injection rates and high concentration of copper ions. This method can be useful for large-scale production of monodispersed copper particles.
Materials and methods
Materials and synthesis
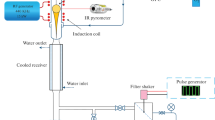
All reagents used in this work were analytical grade, including ethylene glycol (E.G., C2H6O2, M w = 62.078, 99.5%, Carlo Erba) as a protecting agent, hydrazine monohydrate (N2H5OH, %80, M w = 50, 80%, Merck) as a reducing agent and copper (II) sulfate anhydrous (CuSO4, M w = 159.61, Merck) as a copper source. Copper particles were obtained by adding hydrazine monohydrate to the aqueous solution of copper (II) sulfate and E.G. at ambient atmosphere. In a typical preparation of copper particles, 20 mL of an aqueous solution containing 0.32 g (2 mmol) CuSO4 and various amounts of E.G. at 60 °C (Table 1) was titered by NaOH until pH = 4 was reached. Then a 5 mL aqueous solution containing 0.5 mL hydrazine hydrate (80%) was added in various injection rates under ultrasound radiation (Table 1). The synthesized copper particles were recovered from the solution by centrifuging which was subsequently washed repeatedly with distilled water and dried in an oven. X-ray diffraction technique employing Cu Kα1 radiation (1.51059 Å) was used to characterize the composition and crystallinity of synthesized particles. Scanning electron microscopy was employed to reveal the morphology, size, and size distribution of the prepared copper particles.
Characterization of copper nano-particles
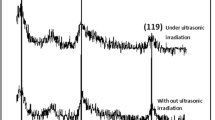
In addition to the particle size and size distribution, phase composition and crystallinity of the synthesized copper particles were investigated by using an X-ray diffractometer. The phase analysis was conducted on the monodispersed copper particles (sample #6). The X-ray diffraction patterns correspond to copper with a crystalline face-centered-cubic (fcc) structure as shown in Fig. 1. The prepared particles were determined to be pure Cu without any impurity such as CuO, Cu2O, and Cu(OH)2. Size and size distribution of synthesized copper particles were determined with Microstructure Measurement software.
Results and discussion
Basic chemical reaction
Typically, during titration of the sodium hydroxide solution, copper (II) sulfate converts to copper hydroxide [Cu(OH)2] followed by the formation of cuprous oxide (Cu2O) as an intermediate product which forms copper as the final product according to the following equation [25]:
Effect of the reducing agent injection rate
Three different injection rates (Table 1) were selected to prepare copper particles at 60 °C. Figure 2 shows the SEM images of synthesized copper particles for different injection rates of the reducing agent. At an injection rate of 0.035 mL/min the average diameter of obtained particles is 898.4 ± 134.1 nm (Fig. 2a). By increasing the injection rate to 0.083 mL/min, smaller particles (296.5 ± 51.2 nm) were obtained with a narrow size distribution but the shape of the particles changed from octahedral and polyhedral type with distinct faces of {111} to a spherical shape (Fig. 2b). Figure 2c shows that a further increase in the injection rate (60 mL/min) results in finer particles with a broader size distribution and the shape of particles changed to irregular shape (192.2 ± 102.4 nm).
The size and size distribution of the obtained particles are determined by the nucleation and the growth mechanisms. The formation of nuclei needs a critical concentration of reduced copper atoms, C crit. The time to reach C crit (Incubation Time (I.T.)) depends on the concentration of reduced copper atoms which is affected by injection rate of the reducing agent, degree of homogenization, and concentration of copper ions. By increasing the injection rate of reducing agent the incubation time will be decreased and also the reduction rate of copper ions exceeds the consumption rate of copper atoms in the growth mechanism. Consequently, the concentration of reduced copper atoms (C saturation) remains over C crit for longer times or fluctuates around it [23] which results in renewed nucleation and polydispersed particles during the reaction (Fig. 4b).
Influence of sonication on the size and size distribution of particles
The effect of sonication on the size and size distribution of particles is described in this section. By employing ultrasound radiation the average diameter of synthesized copper particles decreases from 253.4 ± 74.3 nm (Fig. 3a) to 112.8 ± 19.1 nm (Fig. 3b) by employing ultrasound radiation. These results showed that application of ultrasound radiation results in smaller monodispersed particles compared to the case without using any sonication.
Because of the low dispersibility of the stirrer compared to the ultrasound probe in dispersing reducing agents and reducing copper atoms at high injection rate, local nucleation occurs around the droplet of the reducing agent. This local nucleation increases the local C Saturation and decreases the I.T. around the droplet of the reducing agent. Ultrasound radiation uniformly distributes the reductant through the reaction mixture which decreases the local volumetric concentration (C Saturation) of the reduced copper atoms in compared to the case without using sonication. According to the LaMer diagram, the concentration of copper atoms (C Saturation) which is controlled by reducing agent injection rate, degree of homogenization, and concentration of copper ions determines the size and size distribution of particles. Therefore, in experimental condition, the stage to reach polydispersed particles can be controlled by injection rate, degree of homogenization, and concentration of copper ions. Decreasing the local concentration of copper atoms retards the nucleation stage and consequently increases the incubation time and the number of nuclei (Fig. 4c). When the concentration reaches to C crit, due to the uniform distribution of copper atoms more nuclei form at a short time decreasing the amount of available copper atoms for particle growth per growing particle. Decreasing the amount of available copper atoms (C saturation) for particle growth rapidly drops the volumetric concentration under C crit and stops the nucleation period finally resulting in monodispersed copper particles. After the growth of nucleus starts, smaller particles will be produced due to the large number of nuclei and the lower amount of available copper atoms for growth. Eventually, using high injection rate condition at the presence of the ultrasound radiation produces smaller particles with a narrower size distribution (Fig. 4c).
Schematic representation of size and size distribution of synthesized particles at a low injection rate, b high injection rate of the reducing agent [23], and c with using ultrasound radiation at high injection rate. ((I.T.)b < (I.T.)c and (C saturation)b > (C saturation)c). Increasing the I.T. and decreasing the C saturation under ultrasound radiation results in monodispersed particles
Effect of E.G. concentration as a capping agent
The effect of E.G. concentration on the size and size distribution of particles is described in this section. Ahmadi et al. [26] reported that the size and morphology of Pt particles can be controlled by changing the concentration ratio of capping agent (sodium polyacrylate) to Pt ions. They claimed that the presence of capping agent is believed to have two functions. First, it stops the growth of particles at a small size distribution. Second, it prevents individual colloidal particles to coalescence with each other [26]. Herein the influence of the concentration ratio of E.G. to copper ions on the size and size distribution of copper nano-particles was investigated at the presence of ultrasound radiation. Results show that under ultrasound radiation larger spherical particles were obtained by increasing the concentration ratio of E.G. to copper ions (Fig. 5). We believe that copper ions are covered by E.G. molecules and this layer decreases the reduction rate of copper ion–E.G. structure which inevitably increases the incubation time for copper atoms to reach C crit. Therefore, the nucleation rate of copper particles will be decreased by increasing the concentration of the capping agent (E.G.). Decreasing the nucleation rate elevates the growth rate resulting in larger copper particles. Increasing the concentration of capping agent not only results in larger particles, but also affects on the size distribution of the synthesized particles (Fig. 5). Variation of the average particle size and standard deviation versus concentration ratio of capping agent to copper source has been illustrated in Fig. 6a and b. As it can be seen, the average particle size, increases from 108 to 185 nm at the first interval (samples 5, 6, 7, and 8) and remains constant with a slight variation between 185 and 200 nm at the second interval (samples 8, 9, and 10) and again sharply increases from 200 to 300 nm at the third interval (samples 10 and 11). The increase in the average particle size at these three different intervals are 20, 3.5, and 50 nm per 1 mmol of E.G., respectively. Figure 6b depicts the standard deviation curve in which the maximum standard deviation at sample #10 (8 mmol of E.G.) related to a drop in average size at Fig. 6a. Increase in the average size and decrease in the standard deviation at definite concentrations of E.G. can be related to the amount of E.G. that cover the copper ions. With increasing the amount of E.G. some copper ions (but not all ions) are covered by E.G. causing an increase in the average size and due to unequal distribution of E.G. for copper ions the standard deviation increases. When the concentration of E.G. increases up to definite level, all the copper ions are equally covered by E.G. causing an increase in size of all particles with narrower standard deviation.
SEM images of synthesized copper particles in various amounts of E.G. and size distribution diagrams. a 0 mmol, 108.1 ± 14.1 nm (sample #5); b 1 mmol, 112.8 ± 19.1 nm (sample #6); c 2 mmol, 139.1 ± 24.0 nm (sample #7); d 4 mmol, 185.2 ± 33.9 nm (sample #8); e 6 mmol, 201.7 ± 49.3 nm (sample #9); f 8 mmol, 189.5 ± 59.7 nm (sample #10); and g 10 mmol, 300.2 ± 52.7 nm (sample #11)
Conclusion
We synthesized high pure monodispersed copper nano-particles via a wet chemical route. Results show that increasing the injection rate of reducing agent decreases the size of copper particles and also converts the monodispersed particles to polydispersed form and also affect the particle shape. Monodispersed copper nano-particles with average diameter around 108 nm were obtained by applying ultrasound radiation at higher injection rates of the reducing agent and the concentrated solution. We showed that the concentration ratio of E.G. to copper ions is a key factor in controlling the size of copper nano-particles. Increasing the concentration of E.G. increases the mean diameter of synthesized copper nano-particles. Unlike the polyol method which uses E.G. as solvent, we showed that high pure spherical and monodispersed copper nano-particles can be produced in water at large scale.
References
Gates BC (1995) Chem Rev 95:511
Schmid G (1992) Chem Rev 92:1709
Kamat PV (1993) Chem Rev 93:267
Alivisatos AP (1996) J Phys Chem 100:13226
Beecraft LL, Ober CK (1997) Chem Mater 9:1302
Lisiecki I, Pileni MP (1993) J Am Chem Soc 115:3887
Pileni MP, Nilham BW, Gulik-Krzywicki T, Tanori J, Lisiecki I, Filankembo A (1999) Adv Mater 11:1358
Qi L, Ma J, Shen J (1997) J Colloid Interface Sci 186:498
Yeh MS, Yang YS, Lee YP, Lee HF, Yeh YH, Yeh CS (1999) J Phys Chem B 103:6851
Casella IG, Cataldi TRI, Guerrieri A, Desimoni E (1996) Anal Chim Acta 335:217
Huang HH, Yan FQ, Kek YM, Chew CH, Xu GQ, Ji W, Oh PS, Tang SH (1997) Langmuir 13:172
Vitulli G, Bernini M, Bertozzi S, Pitzalis E, Salvadori P, Coluccia S, Martra G (2002) Chem Mater 14:1183
Liu Z, Bando Y (2003) Adv Mater 15:303
Park BK, Jeong S, Kim D, Moon J, Lim S, Kim JS (2007) J Colloid Interface Sci 311:417
Song X, Sun S, Zhang W, Yin Z (2004) J Colloid Interface Sci 273:463
Liu W, Wang X, Fu S (2006) US Patent 0053927
Lisiecki I, Billoudet F, Pileni MP (1996) J Phys Chem 100:4160
Wu SH, Chen DH (2004) J Colloid Interface Sci 273:165
Shi Y, Li H, Chen L, Huang X (2005) Sci Technol Adv Mater 6:761
Chang Y, Lye ML, Zeng HC (2005) Langmuir 21:3746
Kumar RV, Mastai Y, Diamant Y, Gedanken A (2001) J Mater Chem 11:1209
Dhas NA, Raj CP, Gedanken A (1998) Chem Mater 10:1446
LaMar VK, Dinegar RH (1950) J Am Chem Soc 72:4847
Kim JS, Moon JH, Jeong SH, Kim DJ, Park BK (2007) US Patent 0180954
Miki H (1998) US Patent 5741347
Ahmadi TS, Wang ZL, Green TC, Henglein A, El-Sayed MA (1996) Science 272:1924
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moghimi-Rad, J., Zabihi, F., Hadi, I. et al. Effect of ultrasound radiation on the size and size distribution of synthesized copper particles. J Mater Sci 45, 3804–3811 (2010). https://doi.org/10.1007/s10853-010-4435-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4435-2