Abstract
In this work, results are reported concerning the effect of the Bi concentration on the structural and electrical transport properties of SnS thin films, grown through a chemical reaction of the metallic precursors with elemental sulfur (sulfurization) in a two-stage process. XRD measurements revealed that the samples deposited by sulfurization of Sn or Bi grow in the SnS and Bi2S3 phases, respectively, whereas those obtained by sulfurization of a Sn:Bi alloy grow with a mixture of several phases. Special emphasis was placed on studying through σ versus T measurements, the effect of the Bi concentration on the transport properties of SnS:Bi films. To identify the dominant transport mechanisms, the σ versus T curves were analyzed in two different temperature ranges. It was also found that in the range of temperatures greater than 300 K, the conductivity is predominantly affected by transport of free carriers in extended states of the conduction band, whereas in the range of temperatures below 250 K, the conductivity is dominated by the VRH (variable range hopping) transport mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most extensively studied polycrystalline thin film solar cells are based on CuInSe2 (CIS) and CdTe as absorbent material [1]. Although high efficiency solar cells based on CIS and CdTe have been produced, it is generally recognized that other materials may in the long term have advantages over the former. There is concern respect to the use of cadmium in both types of solar cells but the greatest concern is the supply of indium when the technology of CIS moves to large-scale production. Current efforts are being made in various laboratories to develop new photovoltaic materials with a suitable energy band gap. The factors that should be considered in developing these new materials include: the possibility of depositing them using low cost methods, abundance of the precursor elements, and low environmental impacts. Tin sulfide (SnS) is one of the promising materials for low cost thin film solar cells technology, thanks to its optimum energy band gap Eg and a high fundamental absorption coefficient [2]. Elements Sn and S are abundant in nature and they do not contribute to pollution during the SnS growing process.
Tin sulfide thin films have been deposited by different techniques such as: the vacuum evaporation of the SnS compound [3], two-stage process [4], and electrochemical deposition [5]. In this work, thin films of SnS with donor impurities of Bi have been grown by sulfurization of the precursor species. In general, the SnS films grow with p-type conductivity, however, we have found that substituting Sn by Bi is possible to get n-type SnS:Bi thin films with similar properties to those of the SnS films. This is an interesting result because it allows fabricating p-SnS/n-SnS:Bi cells in situ.
In this work, special emphasis was placed on studying through σ versus T measurements, the effect of the Bi concentration on the transport properties of SnS:Bi films. To identify the dominant transport mechanisms, the σ versus T curves were analyzed in two different temperature ranges. The results revealed that, in the range of temperatures greater than 300 K, the conductivity is predominantly affected by free carrier transport in extended states of the conduction band and in the range of low temperatures, the conductivity is predominantly affected by transport in a band of localized states as predicted by the Davis–Mott model, where the carriers can move between states via a phonon-assisted tunneling process [6]. This transport mechanism is known as variable range hopping (VRH).
Experimental
The SnS:Bi films were grown by sulfurization of the metallic precursors, in a two-stage process. In the first stage, a thin film is deposited by evaporating a mixture of Sn and Bi from the same boat, on a glass substrate at ambient temperature. In the second stage, the film of the Sn:Bi alloy is annealed for 40 min at temperatures ranging from 350 to 450 °C in a sulfur environment, produced by evaporation of elemental sulfur from a tantalum effusion cell, at temperatures around 140 °C. A thickness monitor (Maxtec TM-400) with a quartz crystal as sensor was used to measure the flux of the metallic species, and a PID temperature controller (Eurotherm 900C) was used to control the temperature of both substrate and sulfur evaporation.
It is well known that tin sulfide films can grow in different phases such as SnS, SnS2, Sn2S3, etc. [7]; however, only the SnS phase has p-type conductivity and good photovoltaic properties [8]; therefore, it was necessary to perform an exhaustive study of the synthesis parameters to achieve samples containing only the SnS phase. This study revealed that the parameters most critically affecting the phase in which the samples grow and their properties are the annealing temperature and the amount and flux of evaporated S. The results indicated that it is possible to grow films in the SnS phase by annealing the metallic precursors at temperatures greater than 400 °C, in a sulfur atmosphere obtained by evaporating an adequate amount of S at 140 °C. The samples prepared at annealing temperatures lower than 300 °C grow predominantly in the SnS2 phase.
The SnS films with different Bi concentrations (SnS:Bi) were deposited using the same parameters that allowed us to get tin sulfide films in the SnS phase; the content of Bi in the film was varied by varying the amount of Bi that is added to the evaporated mixture of Sn and Bi. Electrical conductivity measurements were carried out using a procedure based on the two probe method, varying the temperature in a range between 90 and 550 K. The conductivity type of the SnS:Bi films was determined through thermoelectric power measurements and the phase in which the samples grow was determined by XRD measurements using the Cu Kα radiation of a Shimadzu-6000 diffractometer. Film thickness was ~800 nm and these were measured with a Veeco Dektak 150 surface profiler.
Results and discussion
Structural characterization
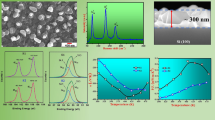
The SnS:Bi films were initially characterized through XRD measurements in order to get information regarding the influence of the Bi content on the phase in which the films grow, as well as on the crystalline structure. Figure 1 shows typical XRD spectra of SnS:Bi films deposited varying the concentration of Bi in the evaporated mixture of Sn and Bi, where the concentration x is determined by the relation: x = [mols of Bi/mols of (Bi + Sn)]*100.
The experimental XRD spectra were analyzed by comparing them with those reported in the JCPDS data base. Theoretical simulations of the XRD spectra of Fig. 1 were also performed using Powder Cell simulation program and the network constants were obtained trough Rietveld refinement method [9, 10], in order to improve the confidence degree of the analysis. In Table 1, are listed the phases identified in the SnS:Bi films whose diffractograms are depicted in Fig. 1; the corresponding structure and lattice parameter are also listed in Table 1. From Table 1, is evidenced a mixture of phases substituting Bi atoms on SnS composite.
The results show that the films deposited through a chemical reaction of Sn or Bi with sulfur grow in the SnS and Bi2S3 phases, respectively, whereas those obtained by sulfurization of a Sn:Bi alloy grow with a mixture of the Bi2S3, SnS2, and SnS if the Bi concentration (in the evaporated material) is greater than 50% and with a mixture of Bi2S3, SnS, and Sn2S3 if the Bi concentration is less than 50%.
Electrical characterization
In Fig. 2, are depicted curves of lnσ versus 1,000/T performed corresponding to SnS:Bi thin films deposited varying the concentration of Bi in the evaporated material, between 0 and 100%.
The measurements show two quite different slopes, indicating that the conductivity is affected by two different transport mechanisms, each one predominating in a different temperature range; on the other hand, it is observed that the electric conductivity of the SnS films at room temperature is significantly reduced by the incorporation of Bi in the SnS lattice (see Fig. 2). This behavior is caused by compensation of the p conductivity of the SnS films, which is induced by the substitution of Sn by Bi donor impurities. It is also observed that the SnS:Bi films with Bi concentrations lower than 50% present values of conductivity lower than those of the samples with higher Bi concentrations, probably due to the presence of the Sn2S3 phase in this type of samples, which is much more resistive than the other phases present in samples with Bi concentrations greater than 50% [11].
From thermoelectric power measurements, it was also found that the SnS:Bi films with Bi concentrations less than 50% present p-type conductivity, whereas the samples with Bi concentrations greater than 50% are n-type. This result indicates that it is possible to fabricate in situ solar cells with p-SnS/n-SnS:Bi structure.
The results of Fig. 2 were analyzed assuming predominance of one determined mechanism in a determinate temperature range in an attempt to identify the mechanisms affecting the electrical transport of the SnS:Bi thin films. From this analysis, it was found that in the range of temperatures greater than 300 K, the conductivity can be expressed by the relation: \( \sigma = \sigma_{0} \exp [ - (E_{\text{C}} - E_{\text{F}} )/(kT)], \) indicating that in this range of temperatures the conductivity is predominantly affected by transport of free carriers in extended states of the conduction band. As the coefficient σ0 (given by qN Cμ(T)) varies slowly with temperature, whereas the term exp[−(E C − E F/kT] strongly depends on T, σ0 can be assumed to be constant. Under this approximation, the slope of the plot of lnσ versus 1/T gives the activation energy E a = E C − E F.
The linear behavior of the lnσ versus 1,000/T curves, demonstrates that the conductivity of the SnS:Bi films in the high temperature range is dominated by transport of free carriers in extended states of the conduction band. The results of Fig. 2 show additionally that the Bi concentration, significantly affects the activation energy of the SnS:Bi samples (see inset of Fig. 2).
In the range of low temperatures (T < 250 K), the curve of lnσ versus T −1/4 can be expressed by a relation of the form:
where σ0 and A are constants depending on the material, and can be experimentally determined. The behavior of σ versus T indicates that in the range of low temperatures, the conductivity is predominantly affected by transport in a band of localized states at the Fermi level, as predicted by the Davis–Mott model, where the carriers can move between states via phonon-assisted tunneling process [6]. This transport mechanism is known as VRH and has been observed in amorphous semiconductors [12] and metal alloys [13].
Figure 3 shows plots of ln[σ(T)] versus T −1/4 performed with the experimental data of Fig. 2. The linear behavior of the ln[σ(T)] versus T −1/4 curves confirms that the conductivity of the SnS:Bi films in the low temperature range is dominated by the VRH transport mechanism.
Based on the classic formalism of the percolation theory, the parameters characterizing the hopping transport (hopping activation energy W and hopping range R) can be determined through the following expressions [14]:
where α−1 varies typically between 3 and 30 Å [15].
Figure 4 shows the variation of the parameters W and R as a function of temperature, for SnS:Bi thin films with different Bi concentrations. α−1 = 14 Å was assumed.
The results of Fig. 4 show that the activation energy W of the VRH transport in SnS:Bi films as well as the hopping range are significantly affected by the Bi concentration. It is also observed in Fig. 4 that the activation energy increases with temperature, whereas the hopping range decreases with temperature. These results indicate that the energy required for a carrier to jump from one localized state to another increases with both the temperature and the increase of the Bi content in the SnS:Bi film. Contrary to this, the jumping distance decreases with temperature.
Conclusions
SnS:Bi thin films were grown through the chemical reaction of the metallic precursors with elemental sulfur evaporated from an effusion cell in a two-stage process. XRD measurements revealed that the films deposited by sulfurization of Sn and Bi grow in the SnS and Bi2S3 phases, respectively, whereas the films deposited by sulfurization of the Sn:Bi alloy grow with a mixture of the Bi2S3, SnS2, and SnS phases if the Bi concentration in the evaporated Sn:Bi alloy is greater than 50% and with a mixture of Bi2S3, SnS, and Sn2S3 if the Bi concentration is less than 50%.
It was found from thermoelectric power measurements that the SnS:Bi films with Bi concentrations lower than 50% present p-type conductivity, whereas the samples with Bi concentrations greater than 50% are n-type. These results indicate that it is possible to fabricate in situ solar cells with p-SnS/n-SnS:Bi structure.
Measurements of conductivity as a function of temperature revealed that the electrical transport in SnS:Bi films is affected by two different mechanisms. In the range of temperatures greater than 300 K, the conductivity is predominantly affected by transport of free carriers in extended states of the conduction band, whereas in the range of temperatures lower than 250 K, the conductivity is dominated by the VRH transport mechanism. It was found that the energy required for a carrier to jump from one localized state to another increases with both the temperature and the increase of the Bi content in the SnS:Bi film.
References
Contreras MA, Egaas B, Ramanathan K, Hiltner J, Swartzlander A, Hasoon F, Noufi R (1999) Prog Photovolt Res Appl 7:311
Frantzis L, Jones E, Lee C, Wood M, Wormser P (2000) Proceedings of 16th European photovoltaic solar energy conference, Glasgow, UK
Meyer N, Luck I, Ruehle U, Klenk R, Lux-Steiner MC, Scheer R (2004) Proceedings of 19th European photovoltaic solar energy conference, Paris, France, p 1698
Kaigawa R, Neisser A, Klenk R, Lux-Steiner MC (2002) Thin Solid Films 415:266
Braunger D, Durr Th, Hariskos D, Koeble Ch, Walter Th, Wiser N, Schock HW (1996) Proceedings of 25th IEEE photovoltaic specialist conference, Washington, p 327
Mott NF (1969) Philos Mag 19:333
Bletskan DI (2005) J Ovonic Res 1:59–67
Nair MTS, Nair PK (1991) Semicond Sci Technol 6:132
Gordillo G, Romero E (2005) Thin Solid Films 484:352–357
Rietveld HM (1969) J Appl Crystallogr 2(2):65
Gordillo G, Botero M, Oyola JS (2008) Microelectron J 39:1351
Adler D, Fritzsche H (1985) Tetrahedrally–bonded amorphous semiconductors. Plenum Press, New York and London
Long AR, Hansmann L (1990) In: Fritzsche H, Pollak M (eds) Hopping and related phenomena. World Scientific Publishing Co., Singapore
Concari SB, Buitrago RH (2004) J Non-Cryst Solids 331–335:331
Hill RM (1971) Philos Mag 24:1307
Acknowledgements
This work was supported by the Universidad Nacional de Colombia and Colciencias.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dussan, A., Mesa, F. & Gordillo, G. Effect of substitution of Sn for Bi on structural and electrical transport properties of SnS thin films. J Mater Sci 45, 2403–2407 (2010). https://doi.org/10.1007/s10853-010-4207-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4207-z