Abstract
Polyaniline films prepared on titanium were employed as substrate for the electrodeposition of gold. The modified electrode was used as anode for the electro-oxidation of ascorbic acid. The electrochemical behavior and electro-catalytic activity of Au/PAni/Ti electrode were characterized by cyclic voltammetry. The morphology of the polyaniline film and gold coating on PAni/Ti electrode were characterized by scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) techniques, respectively. Results indicated that gold nanoparticles were homogeneously dispersed on the surface of polyaniline film. The electro-oxidation of ascorbic acid is found to proceed more facile on Au/PAni/Ti electrode than on bare gold electrode. The irreversible oxidation current of ascorbic acid exhibits a linear dependence on the ascorbic acid concentration in the range of 1–5 mM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conducting polymers are of great interest to researchers in many fields due to their outstanding properties and numerous possible applications. Polyaniline is one of the most important conducting polymers because of its high conductivity, ease of preparation, good environmental stability, and large variety of applications such as electro-chromic devices, secondary batteries, catalysis, and corrosion protection coatings [1–4]. The electro-oxidation of ascorbic acid on various electrodes has been a subject of several studies. The electrode materials include platinum, carbon paste electrodes, polypyrrole nanowire-modified electrode, vanadium oxide polypropylene carbonate-modified electrode, PEDOT-modified electrode, layer-by-layer assembled multilayer films of redox polymers, polypyrrole films containing ferrocyanide ions deposited onto thermally pre-treated and untreated iron substrate, etc., [5–12]. In all these studies, the reaction has been studied on the bare or the surface-modified noble metals as well as glassy carbon. The noble metals and glassy carbon showed good catalytic activity toward oxidation reactions. Study of electro-oxidation of ascorbic acid on surface-modified non-noble electrodes such as titanium, lacking electro-catalytic activity in their original state, is an interesting field of research. Additionally, low cost as well as ease of electrode assembly are favorable factors in using non-noble metals. Because ascorbate sensors are anticipated to have potential applications, it would be beneficial to employ a suitable metal for electro-oxidation of ascorbic acid. To the best of our knowledge, the electro-oxidation of ascorbic acid on a polyaniline-coated non-platinum metals is only reported in two articles in the literature [13, 14]. Immobilization of the noble metal nanoparticles in an active matrix may enhance the overall reactivity of the catalytic metal centers. For example, our previous studies on the electro-catalytic oxidation of glycerol and evolution of chlorine on Pt/TiO2/Ti electrode and electro-catalytic oxidation of glucose on Au/TiO2/Ti electrode, has shown that the modification of electrode surface by anodizing of titanium enhances the electro-catalytic activity to a great extent [15–17]. To date, the variety of researches has been focused on the using of nanoparticles and nanocomposites in the electrochemistry fields [18–21]. The aim of the present work was to investigate the electro-oxidation of ascorbic acid on gold nanoparticles dispersed in polyaniline matrices and analyze the effect of their morphologies on the electro-catalytic characteristics of this modified electrode. The polyaniline films were obtained by applying cyclic voltammetry scans on titanium substrates. Our criteria behind selecting titanium as substrate for electrodeposition of polyaniline are its good corrosion resistance and human body biocompatibility. Gold nanoparticles dispersed on the polyaniline films by cathodic electrodeposition. The electro-oxidation of ascorbic acid on Au/PAni/Ti electrodes was carried out using cyclic voltammetry and the results are discussed on the basis of differences in their surface area. The surface morphology and element analysis of polyaniline films on titanium and gold coating on polyaniline films were characterized by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX), respectively.
Experimental
Solutions and chemicals
All chemicals used were of analytical grade. Aniline was distilled under reduced pressure and then stored at low temperature before use. All electrochemical experiments were carried out at room temperature. Distilled water was used throughout.
Electrochemical instrumentation, cells, and electrodes
The electrochemical experiments (electro-polymerization and electro-oxidation) were performed in a three-electrode cell assembly. A platinum sheet of the geometric area of about 20 cm2 was used as counter electrode, while all potentials were measured with respect to a commercial saturated calomel electrode (SCE). Electrochemical experiments were carried out using a Princeton Applied Research, EG&G PARSTAT 2263 Advanced Electrochemical System run by PowerSuite Software.
Polyaniline growth
Electro-polymerization of polyaniline from an acidic solution were conducted on titanium electrode. Titanium discs were cut from a titanium sheet (purity 99.99%, 1 mm thickness) and mounted using polyester resin. The deposition of conducting polymers on spontaneously passivating metals such as titanium and aluminum usually requires a pre-treatment of the substrate in order to remove natural oxides, which cover the metal surface. Prior to electro-polymerization of aniline, the titanium electrodes were first mechanically polished with different grades of abrasive papers, rinsed in a run of distilled water, then chemically etched by immersing in a mixture of volumetric 1:4:5 of HF:HNO3:H2O. The last step of pretreatment was rinsing with deionized water. After the pretreatment, electro-polymerization of aniline was conducted in solution of 0.1 M aniline and 0.5 M H2SO4 through 10 successive cyclic voltammetry scans in the potential range of −0.5 and 1.5 V at a scan rate of 10 mV s−1.
The electrodeposition of gold on PAni/Ti electrode
After rinsing with water, the PAni/Ti electrodes were immerged into the bath for electrolytic deposition. Deposition of gold on PAni/Ti electrodes was performed under galvanostatic conditions with a current density of 10 mA cm−2 for 10 min, in a bath containing KAu(CN)2 in the presence of a citrate buffer with pH = 4. The temperature is maintained at 45 °C. The morphology of the polyaniline film and gold coating on the polyaniline films were characterized with a Philips scanning electron microscope.
Determination of electrode surface area
The area of the electrode was determined using 1 mM K4Fe(CN)6 in 0.1 M KNO3 electrolyte by recording the cyclic voltammograms. From the cyclic voltammetric peak current and the diffusion coefficient of hexacyanoferrate, the area of the electrode was calculated by using the equation [22, 23]:
where n = number of electrons transferred, i.e., in this case 1, A = surface area of the electrode, D o = diffusion coefficient (9.382 × 10−6 cm2 s−1), ν = scan rate (0.1 V s−1), C *o = concentration of electro-active species (1 mM). The surface area of Au/PAni/Ti electrode was estimated to be about 5 times of Au electrode.
Results and discussion
Preparation of polyaniline electrode
Figure 1 shows cyclic voltammograms recorded during electro-polymerization of polyaniline on titanium electrode in 0.5 M H2SO4 solution containing 0.1 M aniline at a scan rate of 10 mV s−1. First anodic peak occurring at a potential of about +0.25 V could be attributed to the doping of sulfate anions via transition of leucoemeraldine form of polyaniline to emeraldine salt, while further increase of the potential above +0.65 V denotes transition of emeraldine salt to pernigraniline salt [20]. Between these two well-defined anodic peaks, small peak at potential of about +0.45 V could be assigned to degradation reaction of polyaniline [24, 25].
Morphology of PAni/Ti and Au/PAni/Ti electrodes
Figure 2a, b shows SEM micrograph of the surface of titanium substrate before deposition of polyaniline film onto it and SEM picture of the pure polyaniline film (without deposited gold) obtained by CV polymerization in 0.5 M H2SO4 containing 0.1 M aniline, respectively. The potential range was between −0.5 and 1.5 V with a scan rate of 10 mV s−1. It can be seen that Ti-substrate was smooth and planar before depositing the film onto it and the polyaniline film forms with a granular morphology.
Figure 3 shows the SEM micrographs of gold catalyst nanoparticles electrodeposited on the polyaniline film. It can be seen that the gold nanoparticles with diameters about of 60–90 nm are distributed in an almost homogeneous manner at the surface of the polyaniline film. Figure 4 shows the EDX spectrum of Au/PAni/Ti after 10 min electroplating of gold on PAni/Ti electrode. EDX results confirm the presence of gold nanoparticles in the displaced films.
Characterization of the Au/PAni/Ti electrode surface
In order to determine whether the electrodeposition procedure had resulted in the removal of the surface oxide layers, thereby ensuring good electrical contact between the gold and polyaniline coating with the underlying titanium electrode, the Au/PAni/Ti were tested as electrode using an one electron redox couple. Figure 5 shows the voltammetric curves for the reduction of K3Fe(CN)6 on Au/PAni/Ti and Au electrodes. The voltammogram for the Au/PAni/Ti electrode shows the expected reversible behavior for the reduction on a bulk gold electrode. It suggests that the adhesion and electrical contact between electrodeposited polyaniline film and titanium substrate is quite satisfactory.
Electro-oxidation of ascorbic acid
In order to compare Au/PAni/Ti electrode with flat gold electrode, the method of cyclic voltammetry was used to follow the electro-catalytic behavior of the electrodes. Figure 6 presents cyclic voltammograms of gold and Au/PAni/Ti electrodes in 0.1 M phosphate buffer + 0.005 M ascorbic acid aqueous solution, at a scan rate of 100 mV s−1. The pure gold electrode exhibited no oxidation peak for ascorbic acid but after replacing the pure gold electrode with Au/PAni/Ti electrode, distinguished peaks were observed in the cyclic voltammetry, therefore confirming the Au/PAni/Ti electrodes electro-catalytic activity.
Effect of scan rate
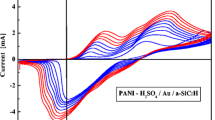
The effect of scan rate on the electro-catalytic properties of Au/PAni/Ti electrode toward ascorbic acid oxidation was studied and results were shown in Fig. 7. As can be seen in Fig. 7, the increase in potential scan rate induced an increase in the electro-catalytic peak current and resulted in a shift to more positive potential value for the catalytic oxidation of ascorbic acid. The same results were observed by other authors concerning the ascorbic acid oxidation on modified carbon paste electrode [26]. This clear shift of the peak potential was occurred as expected for irreversible electrochemical reactions [27]. The obtained cyclic voltammograms were used to examine the variation of oxidation peak current versus scan rate. The oxidation current of ascorbic acid increased linearly with the square root of the scan rate on Au/PAni/Ti electrode (Fig. 8), suggesting that the reaction is mass transfer controlled.
In order to get the information on the rate-determining step, Tafel slope, b, was determined using the following equation valid for such a process [13]:
Therefore, on the basis of Eq. 2, the slope of E p versus log ν plot is
where b is the Tafel slope and ν is the scan rate; the Tafel slope can also be expressed as:
On the basis of these equations, the slope of the plots of E p versus log ν is b/2 which was found equal to 0.0918 in this work (Fig. 9), so, b = 2 × 0.0918 V = 0.1836 V. It is known that ascorbic acid oxidation kinetics on many materials occur with single electron transfer process [28, 29]. Assuming this, these slope values indicate a transfer coefficient (α) of 0.3125.
Electro-catalytic determination of ascorbic acid
Effect of ascorbic acid concentration on the cyclic voltammetric response of Au/PAni/Ti electrode was investigated. Figure 10 shows the cyclic voltammograms of the Au/PAni/Ti electrode at the presence of various concentrations of ascorbic acid. The observed anodic peak current increases with increasing ascorbic acid concentration in the solution. This catalytic peak current shows a linear relationship with the concentration of ascorbic acid in the range of 1–5 mM with a correlation coefficient of r 2 = 0.9924 (Fig. 11). From these results, it can be concluded that the electro-oxidation of ascorbic acid on these new modified electrodes can be used for the quantitative determination of ascorbic acid in samples. Compared to modified carbon electrodes requiring tedious preparations and pretreatment procedures, Au/PAni/Ti electrode can easily be prepared without any further need to modification, thus from a practical point of view can be applied for the quantitative determination of ascorbic acid. The same results were observed by some investigators concerning the ascorbic acid oxidation on polypyrrole films containing ferrocyanide ions deposited onto thermally pre-treated and untreated iron substrate [12].
The temperature dependence of ascorbic acid oxidation on Au/PAni/Ti electrode
In order to study the effect of temperature on the electro-catalytic performance of Au/PAni/Ti electrodes, ascorbic acid oxidation on Au/PAni/Ti electrode was investigated in the electrolyte temperature range of 308–358 K by the method of cyclic voltammetry. From Fig. 12, it can be seen that anodic current increase with temperature. Figure 13 presents the variation of ascorbic acid electro-oxidation current density versus temperature. As the temperature increases, the electro-oxidation current density increases. This is attributed to the increase rate of charge transfer in the electrode/electrolyte interface. At the same time, high temperature will decrease the diffusion resistance, so higher electro-oxidation currents could be obtained.
Conclusion
Au/PAni/Ti electrode was prepared by a two-step process consisting of electro-polymerization of aniline on the titanium electrodes and then, cathodic electrodeposition of gold on polyaniline films (on PAni/Ti electrode). The morphology and electro-catalytic performance of the electrode was investigated by SEM and cyclic voltammetry, respectively. The results indicated that gold nanoparticles were homogeneously deposited on the surface of polyaniline film. These electrodes presented a good electro-catalytic activity toward the oxidation of ascorbic acid. The electro-catalytic activity of the Au/PAni/Ti electrodes and pure gold toward ascorbic acid oxidation was evaluated through cyclic voltammograms. Results showed that PAni/Ti electrodes modified with gold nanoparticles have good electro-catalytic effect toward ascorbic acid electro-oxidation and that the bare gold electrode could not be considered suitable for oxidation of ascorbic acid. The oxidation kinetic of ascorbic acid was also studied by varying the potential scan rate. The results indicated that the oxidation process is mass transfer controlled. Finally, the oxidation current of ascorbic acid Au/PAni/Ti electrodes was used for the determination of ascorbic acid in aqueous solution and a linear calibration curve was found in the range of 1–5 mM with a correlation coefficient of 0.9924. Therefore, this modified electrode can be used for quantitative determination of ascorbic acid in samples.
References
Zhang LJ, Wan MX (2003) J Phys Chem B 107:6748
Liang L, Liu J, Windisch CF, Exarhos GJ, Lin Y (2002) Angew Chem Int Ed 41:3665
Hosseini MG, Sabouri M, Shahrabi T (2006) Mater Corros 57:407
Hosseini MG, Sabouri M, Shahrabi T (2008) J Appl Polym Sci 110:2733
Xing X, Bae IT, Shao M, Liu CC (1993) J Electroanal Chem 364:309
Ormonde ED, O’Neill RD (1990) J Electroanal Chem 279:109
Wang J, Wang J, Wang Z, Wang S (2006) Synth Met 156:610
Tian L, Chen L, Liu L, Lu N, Song W, Xu H (2006) Sens Actuators B 113:150
Bello A, Giannetto M, Mori G, Seeber R, Terzi F, Zanardi C (2007) Sens Actuators B 121:430
Qian L, Gao Q, Song Y, Li Z, Yang X (2005) Sens Actuators B 107:303
Takita R, Yoshida K, Anzai JI (2007) Sens Actuators B 121:54
Oukil D, Makhloufi L, Saidani B (2007) Sens Actuators B 123:1083
Pournaghi-Azar MH, Razmi-Nerbin H (2000) J Electroanal Chem 488:17
Rajendra Prasad K, Munichandraiah N (2002) Anal Chem 74:5531
Hosseini MG, Sajjadi SAS, Momeni MM (2007) Surf Eng 23:419
Hosseini MG, Sajjadi SAS, Momeni MM (2008) IUST Int J Eng Sci 7:39
Hosseini MG, Momeni MM (2009) J Solid State Electrochem. doi:10.1007/s10008-009-0920-4
Jiwei L, Jingxia Q, Miao Y, Chen J (2008) J Mater Sci 43:6285. doi:10.1007/s10853-008-2905-6
Chen W, Ghosh D, Chen S (2008) J Mater Sci 43:5291. doi:10.1007/s10853-008-2792-x
Tu WY, Xu BS, Dong SY, Wang HD, Bin J (2008) J Mater Sci 43:1102. doi:10.1007/s10853-007-2259-5
Zhang DW, Chen CH, Zhang J, Ren F (2008) J Mater Sci 43:1492. doi:10.1007/s10853-007-2274-6
Bard AJ, Faulkner LR (2004) Elecrochemical methods fundamentals and applications, 2nd edn. Wiley, New York
Gosser DK (1994) Cyclic voltametry. VCH, New York
Gospodinova N, Terlemezyan L (1998) Prog Polym Sci 23:1443
Lee JY, Ong LH, Chuah GK (1993) J Appl Electrochem 23:1031
Raoof JB, Ojani R, Rachid-Nadimi S (2004) Electrochim Acta 49:271
de la Fuente C, Acuna JA, Vasquez MD, Tascon ML, Gomez MI, Batanero PS (1997) Talanta 44:685
Mazloum Ardakani M, Akrami Z, Kazemian H, Zare HR (2006) J Electroanal Chem 586:31
Fernandez L, Carrero H (2005) Electrochim Acta 50:1233
Acknowledgements
The authors would like to acknowledge the financial support of Iranian Nanotechnology Society and the Office of Vice Chancellor in Charge of Research of University of Tabriz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, M., Momeni, M.M. & Faraji, M. Electrochemical fabrication of polyaniline films containing gold nanoparticles deposited on titanium electrode for electro-oxidation of ascorbic acid. J Mater Sci 45, 2365–2371 (2010). https://doi.org/10.1007/s10853-009-4202-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-4202-4