Abstract
Outstanding controlled release materials were developed using statistically random copolymers of l-lactide (l-LA) with ε-caprolactone (CL) using Sn(oct)2 as a catalyst at 150 °C for 24 h without solvent. Preparation of novel controlled release materials was carried out using useful organic compounds with low boiling points and synthetic random copolymers composed of l-LA and CL as base materials under supercritical carbon dioxide (scCO2). Low-boiling-point compounds such as d-limonene, hinokitiol, and trans-2-hexenal were used. In impregnation experiments using scCO2, the amounts of low-boiling-point compounds increased with an increase in l-LA content. When enzymatic degradation of poly(l-LA-ran-CL) was performed using lipase PS, copolymers with higher CL contents degraded more rapidly than did copolymers with higher l-LA content. In contrast, enzymatic degradation of copolymers occurred to a higher degree with increased l-LA content in enzymatic degradation by proteinase K. In a controlled release experiment with poly(l-LA-ran-CL) (=73/27), after 400 h of degradation by proteinase K, the remaining weight of the copolymer pellet was 6% and the amount of d-limonene remaining in the pellet was 15%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The purpose of this study was to develop a material that allows controlled release of an insect repellent or antibacterial agent via hydrolysis of a biodegradable polymer. In recent years, increasing damage has been caused by insect pests and animals on farms and in other areas. This has become a serious problem resulting in the need for time-consuming labor, such as the spraying of agricultural chemicals or insect-repellents. Although there has been a great deal of research on various methods of extermination [1, 2], materials whose effects can be maintained over a long period have not yet been reported. In the field of medicine, biodegradable polymers that can maintain therapeutic effects in humans over long periods have been investigated; there have been many reports of the controlled release of substances by biodegradable polymers as drug delivery systems (DDS) [3–7].
For the production of a DDS, solutions of a polymer and a drug are emulsified for joint distribution. The resulting material can be prepared in microcapsule form. However, since most insect repellents and antibacterial agents are highly volatile organic compounds, this method usually cannot be used for such substances. The methods generally used to impregnate a polymer with a target organic compound are mulling and solvent dissolution. However, several problems are associated with these methods. Although high-boiling-point compounds can be incorporated into a polymer using the mulling method, compounds with low boiling points, which volatilize at the processing temperature, cannot be treated in this way. At the same time, the solvent dissolution method is subject to problems such as volatilization of agents with low boiling points at the time of solvent removal and the presence of residual organic solvents. Rather than using these general methods, we decided to investigate a method of impregnation using supercritical carbon dioxide (scCO2) as a solvent. Supercritical fluids, as well as a large number of organic solvents, are generally utilized as hydrophobic solvents. In particular, scCO2 does not damage treated compounds, is non-toxic, and is more environmentally friendly than are other solvents [8, 9]. Furthermore, the advantage of using scCO2 is the ability to impregnate polymers with targeted compounds without changes of form (film, net, nonwoven fabric, etc.). As a medium that dissolves and softens polymers, scCO2 is used for the foaming of polymers [10–13] and the preparation of microcapsules [14–18].
In this work, poly(l-LA), in the form of a pellet or films, was initially used as a base material. The impregnation experiment was conducted under scCO2 using d-limonene, which is a terpene hydrocarbon with a highly repellent effect on undesirable insects, and hinokitiol and trans-2-hexenal as antibacterial agents. However, after 3 h, only 2.2% of d-limonene was incorporated in poly(l-LA) film, a biocompatible biodegradable semicrystalline polymer, under scCO2 (40 °C, 20 MPa). To improve the characteristics such as hardness and brittleness, l-LA copolymers have been synthesized using various lactones [19–23]. In this work, a copolymer of l-LA with ε-caprolactone (CL) was used as a base material to improve the impregnation content and controlled release of volatile compounds. The impregnation experiment was conducted under scCO2 using d-limonene, hinokitiol, and trans-2-hexenal. The degradability and the controlled release of the impregnated polymers were evaluated by enzymatic degradation.
Experimental
Materials
l-Lactide (l-LA) [(3S)-cis-3,6-dimethyl-1,4-dioxane-2,5-dione] (Aldrich) was purified by recrystallization from THF followed by sublimation at 80 °C. ε-Caprolactone (CL) (Wako Pure Chem. Ind., Ltd.) was purified by distillation under reduced pressure. Tin 2-ethyl-hexanoate [Sn(oct)2] (Sigma) was used as a catalyst without purification. Ethanol and chloroform were purchased from Katayama Chem. Ind. Co., Ltd. Films of synthetic copolymers were prepared by the solvent casting method. d-Limonene, hinokitiol, and trans-2-hexenal were purchased from Wako Pure Chem. Ind., Ltd. and used as received. Lipase PS from Burkholderia cepacia (Wako Pure Chem. Ind., Ltd.) in phosphate buffer, disodium hydrogen phosphate, and sodium dihydrogen phosphate dihydrate at pH 7.0, and proteinase K from Tritirachium album (Wako Pure Chem. Ind., Ltd.) in tricine buffer, N-[tris(hydroxymethyl)methyl]-glycine, at pH 8.0, were used at 37 °C without further purification. Disodium hydrogen phosphate and sodium dihydrogen phosphate dihydrate were purchased from Wako Pure Chem. Ind., Ltd. Tricine was purchased from Nacalai Tesque, Inc. Ion-exchange water was used for degradation tests.
Synthesis of poly(l-lactide-ran-ε-caprolactone)
All polymerizations were carried out under an argon atmosphere using standard Schlenk techniques. Polymerization was performed at 150 °C. Tin 2-ethyl-hexanoate [Sn(oct)2] was used as a catalyst. Sn(oct)2 (0.1 mol% of monomer) was added to a mixture of l-LA and CL in a 20 mL Schlenk tube at room temperature. Polymerization was carried out for 24 h (Scheme 1). The resulting mixture was dissolved in chloroform and then poured into excess methanol in order to precipitate the resulting polymers. The polymer was dried in vacuo.
Incorporation of useful compounds into poly(l-LA-ran-CL) using supercritical carbon dioxide
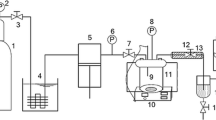
Poly(l-LA-ran-CL) was used in the form of a film (thickness, 100 μm). The polymer films were prepared by the solvent casting method at room temperature. Impregnation of poly(l-LA-ran-CL) with d-limonene and other compounds was carried out inside a pressure-resistant container made of stainless steel (0.5 L) with agitation (100 rpm) under scCO2 (40–75 °C, 20 MPa), using supercritical carbon dioxide fluid equipment (AKICO). The impregnation experiments were conducted under different conditions, varying the concentration of the compound to be incorporated, the temperature, and time. After a predetermined processing time, decompression was gradually carried out over 3 h and the sample was then removed from the container and weighed. Since the sample contained large amounts of CO2 after impregnation, the contents of d-limonene and other compounds were determined by 1H NMR spectroscopy. The amount present was calculated, and the calculated value was used to evaluate the experimental value. The impregnation experiment was conducted three times.
Characterization of polymers
The number- and weight-average molecular weights of the synthetic copolymers were determined by gel permeation chromatography (GPC) using a Hitachi D-2520 chromatograph equipped with Shodex GPC K-802.5 and K-804 columns, using chloroform as an eluent, at 40 °C. The flow rate was 1.0 mL/min. The copolymer was detected with a refractive index detector (RI). The molecular weights were determined with reference to a polystyrene standard.
The thermal characteristics (melting point Tm, glass transition point Tg, and heat of fusion −ΔHm) were obtained using a Rigaku Thermo Plus 2/DSC8230 differential scanning calorimeter. Samples (5 mg) were heated at a rate of 10 °C/min from −100 to 200 °C in a nitrogen stream. Tm and −ΔHm were determined in the first heating and Tg in the second heating.
The compositions of l-LA with CL were determined by 1H NMR spectroscopy using a JEOL JNM-ECP 400 MHz spectrometer. The contents of the organic compounds incorporated into the polymers were also determined by 1H NMR spectroscopy. Chemical shifts were determined relative to residual chloroform at 7.27 ppm.
Degradation and controlled release of poly(l-LA-ran-CL)
Lipase PS from Burkholderia cepacia (activity, 30 IU/mg) and proteinase K from Tritirachium album (activity, 25 IU/mg) were used as enzymes for degradation of poly(l-LA-ran-CL). In a 50-mL sample tube, the enzyme was dissolved in phosphate buffer at pH 7.0 (for lipase PS) and tricine buffer at pH 8.0 (for proteinase K), at 1 IU/mg polymer. The bottle was then placed in a shaking water bath for about 15 min until it reached the degradation temperature of 37 °C. The polymer film (ca. 30 mg) prepared by the solvent casting method at room temperature was sealed in a net bag made from polyethylene sheeting (1 mm × 1 mm mesh), which was placed in the sample tube containing the enzyme and buffer. The enzymatic degradation test was carried out at 37 °C with shaking (100 rpm). The enzyme was replaced every 40 h to ensure that enzymatic activity was maintained at the desired level. After the set incubation time, the samples were washed thoroughly with deionized water and dried in vacuo for 3 h. Evaluation of the degradability was based on the remaining weight of the samples.
The controlled release test was carried out using the same method as the enzyme degradation test. Poly(l-LA-ran-CL) (73/27) impregnated with a compound such as d-limonene was used as the sample. The quantity of the compound remaining in the poly(l-LA-ran-CL) sample was measured by 1H NMR spectroscopy during the test to evaluate the controlled release properties of the material.
Results and discussion
Synthesis of poly(l-lactide-ran-ε-caprolactone)
Copolymerization of l-LA with CL was carried out using Sn(oct)2 as a catalyst (0.1 mol% of monomer) in order to improve upon the content of useful compounds and the controlled release properties of poly(l-LA). Table 1 shows the results of copolymerization at various temperatures. At 150 °C, copolymers were obtained in over 80% yield and over 3.00 × 104 in molecular weight. Composition of the copolymer was almost the same as the feed ratio of l-LA with CL. When the polymerization temperature was lowered to 120 °C, the yield was almost the same, but the molecular weight was cut in half. The l-LA content of the obtained copolymer increased slightly compared to the l-LA feed ratio. At 90 °C, both the yield and the molecular weight decreased remarkably. Furthermore, the l-LA content increased considerably: with a feed ratio of 60 mol% l-LA, a copolymer with 92 mol% of l-LA content was obtained. From these results, it was determined that bulk copolymerization of l-LA with CL using Sn(oct)2 gave good results in terms of yield, molecular weight, and composition at higher temperatures. Subsequent copolymerizations were conducted at 150 °C.
The results of the copolymerization of l-LA with CL at 150 °C are shown in Table 2. At a feed ratio of 10/90, poly(l-LA-ran-CL) (=12/88) was produced in 83.7% yield with a molecular weight of 3.23 × 104. Although copolymerization of l-LA with CL at a 50/50 feed ratio gave the lowest yield (74.5%), the yields improved with an increase in either l-LA or CL. Similarly, the molecular weight was the lowest at a 50/50 feed ratio; almost all other copolymers had molecular weights of 3.00 × 104. The composition ratio of the obtained copolymers was almost the same as the feed ratio. These synthetic copolymers were used as base materials in impregnation experiments of useful organic compounds with low boiling points using scCO2.
Impregnation of poly(l-LA) with useful compounds under supercritical carbon dioxide
The impregnation of poly(l-LA) film (100 μm, 0.3 g) with antibacterial agents trans-2-hexenal and hinokitiol, along with d-limonene, was investigated using 2.0 g for each compound. The results are shown in Fig. 1. The contents of d-limonene, trans-2-hexenal, and hinokitiol in the resulting materials were 1.20, 1.25, and 2.41%, respectively. The value obtained for trans-2-hexenal was almost same as that for d-limonene; it is thought that both compounds show comparable solubility in scCO2. In contrast, the content of hinokitiol was twice that of the other compounds. Since the properties of scCO2 are comparable to those of hydrophobic organic solvents [24–26], it is thought that a highly hydrophobic compound such as hinokitiol should easily dissolve in scCO2 and therefore be more easily incorporated into plasticized poly(l-LA).
Impregnation of poly(l-LA-ran-CL) with d-limonene using supercritical carbon dioxide
As described above, after the process was carried out using a poly(l-LA) film (0.3 g) and 2.0 g of d-limonene under scCO2 (40 °C, 20 MPa) for 3 h, the resulting d-limonene content was 1.20%. In order to impregnate with a higher content of d-limonene, we studied the polymer construction, d-limonene amount, and temperature.
In this experiment, impregnation under scCO2 was carried out using poly(l-LA-ran-CL), so as to increase the d-limonene content. It is known that the melting points (Tm) and glass transition points (Tg) of poly(l-LA) decrease by copolymerization with CL, causing softening of the polymer (Table 3) [20, 23]. Tm and Tg exist only as one value, each, suggesting a random structure for this polymer (Fig. 2). This softening should enable increased levels of impregnation. The impregnation of poly(l-LA-ran-CL) with d-limonene was investigated using 2.0 g under scCO2 (40 °C, 20 MPa) for 3 h. The results of the experiment are shown in Fig. 3. Since the melting point was as low as 50 °C or even lower (as shown in Table 3), copolymers with 34 mol% or less of l-LA were dissolved under scCO2 at 40 °C and 20 MPa, yielding a lower d-limonene content (<1%) than that of poly(l-LA) (1.2%). Copolymers with 66 mol% or more of l-LA did not dissolve under the scCO2 and the d-limonene contents were remarkably higher than were those of poly(l-LA), reaching a maximum with poly(l-LA-ran-CL) (=85/15) of 3.19%. Based on the above results, the d-limonene contents of the copolymers with l-LA ranging from 66 to 95 mol% increased 2–2.7 times compared to that of poly(l-LA).
In order to increase the content of d-limonene further, the d-limonene concentration and temperature were varied in the impregnation experiment. The results of the experiment using poly(l-LA-ran-CL) (=76/24) are shown in Table 4. When 2.0 g of d-limonene (40 °C, 20 MPa, 3 h) was used, the resulting d-limonene content was 2.80%. However, when the amount of d-limonene was increased to 10.0 g, the d-limonene content (6.19%) increased by a factor of 2.2. Increasing the temperature to 75 °C resulted in a d-limonene content of 5.81%. Therefore, increasing the amount of d-limonene and/or the process temperature results in improved levels of d-limonene content in the copolymer.
Impregnation of poly(l-LA-ran-CL) with antibacterial agents using supercritical carbon dioxide
Hinokitiol, trans-2-hexenal, trans-3-hexenal, and cis-3-hexenal are antibacterial agents contained in Japanese cypress, tea leaves, etc. Impregnation experiments were carried out next using trans-2-hexenal and hinokitiol under scCO2.
In this work, poly(l-LA-ran-CL) in the form of a film (100 μm) was used as the base material. Impregnation of poly(l-LA-ran-CL) with trans-2-hexenal or hinokitiol was carried out inside a pressure-resistant container made of stainless steel (0.5 L) with agitation (100 rpm) under scCO2 (40 °C, 20 MPa). After the predetermined processing time, decompression was gradually carried out over 3 h and the sample was then removed from the container and weighed. Since large amounts of CO2 were contained in the sample after impregnation, the contents of trans-2-hexenal or hinokitiol were determined by 1H NMR spectroscopy.
The results of the impregnation of poly(l-LA-ran-CL) with trans-2-hexenal are shown in Table 5. The trans-2-hexenal content of poly(l-LA-ran-CL) (=50/50) was only 0.86%. However, the content of trans-2-hexenal increased as the l-LA content increased, reaching a maximum with poly(l-LA-ran-CL) (=92/8) of 2.56%. Nevertheless, this is low in comparison to d-limonene (3.11% as shown in Fig. 3). trans-2-Hexenal has a lower solubility under scCO2 than does d-limonene, which could be the reason for this result.
The results of the impregnation of poly(l-LA-ran-CL) with hinokitiol are shown in Table 6. In the impregnation of poly(l-LA) with hinokitiol under scCO2 (40 °C, 20 MPa) for 3 h, the hinokitiol content of 2.41% was 2 times greater than that of d-limonene (1.20%), suggesting that the solubility of hinokitiol in scCO2 is higher. The hinokitiol contents of the copolymers increased with an increase in CL content, reaching a maximum with poly(l-LA-ran-CL) (=76/24) of 7.23%. These results demonstrate that incorporation of useful compounds into a copolymer using scCO2 is affected by their solubility under scCO2 and their compatibility with the copolymers.
Degradation of poly(l-LA-ran-CL)
Enzymatic degradation testing of poly(l-LA-ran-CL) was carried out in order to evaluate its capacity for controlled release. Lipase PS and proteinase K were used as enzymes for the enzymatic degradation test. Polymer films (ca. 30 mg) prepared by the solvent casting method were used. The enzyme was dissolved in phosphate buffer at pH 7.0 (for lipase PS) and tricine buffer at pH 8.0 (for proteinase K) at 1 IU/mg polymer.
Figure 4 shows the results of the enzymatic degradation test of poly(l-LA-ran-CL) with lipase PS. It was previously reported that a polyester having a relatively long methylene chain such as poly(butylene succinate adipate) (PBSA) is highly degradable by lipase PS [27]. In this work, the poly(CL), which contains longer methylene chains than does PBSA, was completely degraded after 20 h. The degradation rate decreased with an increase in l-LA content. Consequently, poly(l-LA) was only slightly degraded even after 240 h.
The results of the enzymatic degradation test by proteinase K for poly(l-LA-ran-CL) are shown in Fig. 5. It was previously reported that poly(l-LA) is highly degradable by proteinase K [28–31]. In this work, poly(l-LA) degraded to 7% after 240 h. Poly(l-LA-ran-CL) (=85/15) was easily degraded and disappeared after 60 h. The enzymatic degradability of poly(l-LA-ran-CL) with proteinase K was lowered with an increase in CL content, the opposite of that with lipase PS. The degradation rate of poly(l-LA-ran-CL) (=92/8) was lower than that of random copolymers containing 85 mol% or 76 mol% of l-LA due to the higher crystallinity of the copolymer (=92/8), which is similar to that of poly(l-LA).
Controlled release from poly(l-LA-ran-CL)
Next, enzymatic degradation of a pellet of poly(l-LA) impregnated with d-limonene was carried out, and the quantity of d-limonene remaining in the pellet was measured by 1H NMR. The result of this controlled release test is shown in Fig. 6, with the amount of d-limonene represented by the broken line. After 400 h of degradation by proteinase K, the remaining weight of the poly(l-LA) pellet was 42%, and the amount of d-limonene remaining in the pellet was 40%. This result confirmed that degradation of poly(l-LA) is accompanied by a decrease in the incorporated d-limonene, demonstrating that controlled release of d-limonene had occurred.
Incorporating an insect repellent or antibacterial agent for gradual release via degradation of a biodegradable poly(l-LA-ran-CL) is our ultimate goal. The d-limonene remaining in the copolymer pellet after enzymatic degradation was measured by 1H NMR spectroscopy. The sample (4 mm3 cube) used for the controlled release test was poly(l-LA-ran-CL) (=73/27) containing 3.07% of d-limonene. The results of the enzymatic degradation test using proteinase K and the controlled release test are shown in Fig. 7. The solid line indicates the degradation curve of the copolymer and the broken line indicates the release curve of d-limonene. The degradation rate of the copolymer pellet was slower than that of the poly(l-LA-ran-CL) (=76/24) film (disappearance after 80 h), and the remaining weight was 6% after 400 h. Compared to the film, the copolymer pellet degraded more slowly due to the smaller surface area of the pellet. Furthermore, the relationship between the weight remaining and degradation time gave linear plots for degradation of the copolymer pellet. On the other hand, the degradation line of the copolymer film in Fig. 5 was a smooth curve, indicating that degradation of the pellet proceeded gradually from the surface of the pellet.
The controlled release was evaluated by the remaining d-limonene content measured by 1H NMR spectroscopy after a predetermined degradation time. After 40 h, the weight of the remaining copolymer was 88%, but the content of the remaining d-limonene was 68% (Fig. 7). The weight of the remaining copolymer decreased linearly with degradation time. However, the amount of remaining d-limonene decreased dramatically for the first 80 h and then decreased smoothly thereafter. This is because the d-limonene was released more readily at the surface of the copolymer. In this work, the remaining weight of the poly(l-LA) pellet and d-limonene were 47% and 40%, respectively, after 400 h of enzymatic degradation with proteinase K. Additionally, the remaining weight of the poly(l-LA-ran-CL) (=73/27) pellet and d-limonene were 6% and 15% after 400 h, respectively. From these results, we can conclude that the degradability and the controlled release of poly(l-LA-ran-CL) are superior to that of poly(l-LA).
Conclusions
In the present study, impregnation of poly(l-LA-ran-CL) with d-limonene and other compounds, which are low-boiling-point organic compounds, was carried out inside a pressure-resistant container made of stainless steel under scCO2 (40–75 °C, 20 MPa). Although it was difficult to incorporate organic compounds with low boiling points into the polymer, these compounds could be included in poly(l-LA-ran-CL) using scCO2. This resulted in the production of a novel controlled release material.
The copolymerization of l-LA with CL was carried out at 150 °C using Sn(oct)2 as a catalyst. The molecular weight was the lowest at a 50/50 feed ratio; almost all other copolymers had molecular weights over 3.00 × 104. The composition ratio of the obtained copolymers was almost the same as the feed ratio.
The process was carried out using a poly(l-LA) film (0.3 g) and 2.0 g of d-limonene under scCO2 (40 °C, 20 MPa) for 3 h; the resulting d-limonene content was 1.20%. In order to impregnate with a higher content of d-limonene, impregnation under scCO2 was carried out using poly(l-LA-ran-CL). The d-limonene contents were remarkably higher than with that of poly(l-LA), reaching a maximum with poly(l-LA-ran-CL) (=85/15) of 3.19%.
In order to increase the content of d-limonene further, the d-limonene concentration and temperature were varied in the impregnation experiment. When 2.0 g of d-limonene(40 °C, 20 MPa, 3 h) was used, the resulting d-limonene content was 2.80% for poly(l-LA-ran-CL) (=76/24). However, when the amount of d-limonene was increased to 10.0 g, the d-limonene content (6.19%) increased by a factor of 2.2. Increasing the temperature to 75 °C resulted in a d-limonene content of 5.81%.
In the impregnation of poly(l-LA-ran-CL) with trans-2-hexenal or hinokitiol, the trans-2-hexenal content of poly(l-LA-ran-CL) (=50/50) was only 0.86%. However, the content of trans-2-hexenal increased with an increase in l-LA content, reaching a maximum with poly(l-LA-ran-CL) (=92/8) of 2.56%. In the impregnation of poly(l-LA) with hinokitiol, the hinokitiol content of 2.41% was 2 times greater than that of d-limonene (1.20%), suggesting that the solubility of hinokitiol in scCO2 is higher. The hinokitiol contents of the copolymers increased with an increase in CL content, reaching a maximum with poly(l-LA-ran-CL) (=76/24) of 7.23%.
When enzymatic degradation of poly(l-LA-ran-CL) was performed using lipase PS, the poly(CL) was completely degraded after 20 h. The degradation rate decreased with an increase in l-LA content. Consequently, poly(l-LA) was only slightly degraded even after 240 h. In enzymatic degradation using proteinase K, poly(l-LA-ran-CL) (=85/15) was easily degraded and disappeared after 60 h. The enzymatic degradability of poly(l-LA-ran-CL) with proteinase K was lowered with an increase in CL content, the opposite effect of that with lipase PS.
In a controlled release experiment with poly(l-LA), after 400 h of degradation by proteinase K, the remaining weight of the poly(l-LA) pellet was 42%, and the amount of d-limonene remaining in the pellet was 40%. After 40 h, the remaining weight of poly(l-LA-ran-CL) (=73/27) was 88%, but the remaining content of d-limonene was 68%. The remaining weight of the copolymer decreased linearly with degradation time. However, the amount of remaining d-limonene decreased dramatically for the first 80 h and then decreased smoothly thereafter.
References
Vasuki V, Lalji M, Prasad MP, Kalyanasundaram M (1999) Entomon 24:49
Anthony C, Tigner JR (1968) Wire Wire Prod 43(2):72
Liu R, Ma GH, Wan YH, Su ZG (2005) Colloids Surf B Biointerfaces 45:144
Liu R, Ma GH, Meng FT, Su ZG (2005) J Control Release 103:31
Pamujula S, Graves RA, Kishore V, Mandal TK (2004) Eur J Pharm Biopharm 57:213
O’Donnell PB, McGinity JW (1997) Adv Drug Deliv Rev 28:25
Arshady R (1991) J Control Release 17:1
Hile DD, Pishko MV (2004) J Polym Sci [A] 39:562
Ihata O, Kayaki Y, Ikariya T (2004) Angew Chem Int Ed 43:717
Areerat S, Funami E, Hayata Y, Nakagawa D, Ohshima M (2004) Polym Eng Sci 44:1915
Areerat S, Nagata T, Ohshima M (2002) Polym Eng Sci 42:2234
Koga T, Seo YE, Hu X, Shin K, Zhang Y, Rafailovich MH, Sokolov JC, Chu B, Satija SK (2002) Euro Phys Lett 60:559
Shieh YT, Su JH, Manivannan G, Lee PHC, Sawan SP, Spall WD (1996) J Appl Polym Sci 59:695
Wang Y, Pfeffer R, Dave R, Enick R (2005) AIChE J 51:440
Thote AJ, Gupta RB (2005) Nanomedicine 1:85
Hile DD, Pishko MV (2004) Drug Deliv 11:287
Mishima K, Matsuyama K, Tanabe D, Yamauchi S, Young TJ, Johnston KP (2000) AIChE J 46:857
Ghaderi R, Artursson P, Carlfors J (2000) Eur J Pharm Sci 10:1
Vion JM, Jerome R, Teyssie P, Aubin M, Prud’homme RE (1986) Macromolecules 19:1828
Vanhoorne P, Dubois Ph, Jerome R, Teyssie Ph (1992) Macromolecules 25:37
Kurcok P, Penczek J, Franek J, Jedlinski Z (1992) Macromolecules 25:2285
Zhong Z, Yu D, Meng F, Gan Z, Jing X (1999) Polym J 31:633
Maeda Y, Nakayama A, Arvanitoyannis I, Kawasaki N, Hayashi K, Yamamoto N, Aiba S (2000) Polym J 32:307
Jessop PG, Ikariya T, Noyori R (1999) Chem Rev 99:475
Reverchon EJ (1997) Supercrit Fluids 10:1
Kendall JL, Canelas DA, Young JL, De Simone JM (1999) Chem Rev 99:543
Tsutsumi C, Hayase N, Nakagawa K, Tanaka S, Miyahara Y (2003) Macromol Symp 197:431
Tsutsumi C, Yasuda H (2001) J Polym Sci [A] 39:3916
Tsutsumi C, Nakagawa K, Shirahama H, Yasuda H (2002) Macromol Biosci 2:223
Williams DF (1981) Eng Med 10:5
Reeve MS, McCarthy SP, Downey MJ, Gross RA (1994) Macromolecules 27:825
Acknowledgement
The authors are grateful to the head of the College and the Research Institute for permitting publication of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsutsumi, C., Sakafuji, J., Okada, M. et al. Study of impregnation of poly(l-lactide-ran-ε-caprolactone) copolymers with useful compounds in supercritical carbon dioxide. J Mater Sci 44, 3533–3541 (2009). https://doi.org/10.1007/s10853-009-3477-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3477-9