Abstract
We report preparation of highly photoluminescent CdSe@ZnS quantum dot-polystyrene composite beads, by employing stepwisely: (1) a simultaneous ZnS shell formation and ligand exchange with 3-mercaptopropyltrimethoxy silane (MPS); (2) coupling MPS with polymerizable 3-(trimethoxysily)propylmethacrylate (MPM); and (3) polymerization of the resulting MPM–MPS capped CdSe@ZnS quantum dots with styrene molecules. The functionalized quantum dots exhibited robust chemical stability against the harsh radical polymerization condition and the resulting polymer microbeads were strong against photobleaching under an intense and continuous laser.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, there has been much attention to synthesis and application of inorganic–organic composite materials. Incorporation of quantum dots (QDs) in organic solid matrices is attractive for fabrication of optical bulk materials that exhibit the outstanding luminescent properties of the QDs. Among the luminescent composites, QD-based polymer microbeads are interesting in a broad range of biomedical applications based on the optical encoding and high-throughput analysis of biological molecules [1]. In incorporation of the QDs in polymers, intermolecular forces can be utilized to assemble a functional molecular layer usually on TOPO-capped QDs, which promotes dispersion of QDs in the polymers [2–5].
In an alternative strategy, covalent linking between the QDs and polymer hosts can provide a permanent inclusion and long-term stability to the QDs as well as a direct contact of host materials to the QDs [6]. This relies on functional modification of capping molecules that will coordinate the QDs during the QD preparation process. When commonly available coupling agents such as multifunctional organosilanes and thiols are employed, however, the required postsynthetic ligand-exchange step often degrades the optical property of the QDs rather significantly [7–9].
In our recent work, we have demonstrated that such degradation of QD quality can be avoided when successful ligand exchange is achieved simultaneously in the reaction where CdSe QDs are coated with ZnS shell [10]. The new process afforded highly photoluminescent CdSe@ZnS core/shell QDs capped by 3-mercaptopropyltrimethoxy silane (MPS). In particular, the MPS-capped CdSe@ZnS QDs were chemically stable so that in the subsequent process they could be crosslinked with other silane molecules through a conventional sol–gel process, which resulted in large QD-silica composite monoliths that conserved the original optical properties of the QDs [10]. As an extension of the new approach, we report a straightforward two-step method that provides polymerizable QDs through all covalent functionalization with commercially available organosilane coupling molecules and we demonstrate its efficacy through preparation of highly fluorescent and photostable QD-polystyrene (PS) microbeads.
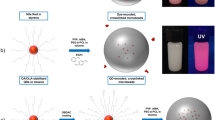
The overall procedure of our experiments is illustrated in Scheme 1. First, CdSe QDs are functionalized with MPS during ZnS passivation, as in our previous QD-silica monolith preparation [10]. Second, the organosilanes on the surface of the resulting CdSe@ZnS QDs are coupled with another type of organosilane molecule that has a polymerizable functional group. 3-(Trimethoxysily)propylmethacrylate (MPM) was used as the organosilane-coupling agent with a polymerizable vinyl group. Finally, QD-polystyrene microbeads are formed via copolymerization of the QDs into polymer matrix.
Experiment
Synthesis of oleylamine-capped CdSe QDs
The synthesis of CdSe core QDs followed a microwave-assisted method reported recently by us [11–13]. All the reactions were loaded in a glove box filled with nitrogen gas and reaction mixtures were taken out from the glove box and heated in air-tight reaction vessels by employing a microwave reactor (CEM Discover System) at 2.45 GHz. All the organic chemicals were purchased form Sigma-Aldrich and used without further purification. B2Se3 was prepared from typical solid-state reactions of the elements in carbon-coated and evacuated silica ampules. Three kinds of oleylamine-capped CdSe QDs were obtained with green, orange, and red photoluminescence (PL) colors. In a typical synthetic process, 0.2 mmol of B2Se3 and 0.6 mmol of CdCl2 (99%, Alfa Aesar) were loaded into 3 mL of oleylamine in separate sealed vials and dissolved by heating at 110 °C for 20 min. After the solutions were cooled down, they were mixed together and divided into three 15-mL crimp-top microwave-reaction vials. The vials were tightly sealed using an aluminum crimp cap with Teflon-lined rubber septum before taken out from the glove box. Each mixture solution was heated at 70, 120, or 190 °C for 60 s in a CEM microwave reactor, and the different reaction temperatures provided products of different PL emission wavelengths (λmax = 540, 584, and 614 nm).
Synthesis of MPS-capped CdSe@ZnS core/shell QDs
The CdSe@ZnS core/shell QDs with MPS as a capping ligand were prepared from the oleylamine-capped CdSe QDs prepared in the first step. The reaction setup followed the synthesis of the oleylamine-capped CdSe QDs. In a typical reaction, 0.1 mmol of P2S5 and 1.2 g of MPS were heated at 110 °C for 20 min in 10 mL of 1-methyl-2-pyrrolidinone (NMP) (99.5%, Aldrich) in a sealed vial that also contained 0.5 mL of butylamine to dissolve the sulfide. NMP has a high boiling point (202 °C) and dissolves the chemical reagents well in our experiment. In a separate vial, 0.5 mmol of ZnCl2, 1.2 g of MPS and 0.5 mL of butylamine were dissolved in 10 mL of NMP and heated in the same way. After cooled down to room temperature, the ZnCl2 solution was divided into five crimp-top vials. About 10 mg of oleylamine-capped CdSe core particles were dissolved in the P2S5 solution and the whole solution was divided into five portions. Each portion of the CdSe/P2S5 solution was injected into one ZnCl2 solution preheated at 70 °C and the solution was kept at the same temperature for 30 s in the microwave reactor, which provided MPS-capped CdSe@ZnS core/shell products. The solution was concentrated by solvent extraction with hexanes.
Synthesis of MPM–MPS-capped CdSe@ZnS core/shell QDs
In a typical reaction, 1 mL of a concentrated solution of MPS-capped CdSe@ZnS QDs was dispersed in a mixture of 70 mL of methanol and 7 mL of H2O and 2 mL of ammonia solution (28–30 wt%), followed by adding 2 mL of MPM. After stirring for 10 h, the MPM–MPS-capped CdSe@ZnS QDs precipitated from the reaction solution. In order to test the role of the polymerizable methacrylate group in MPM in the process, we carried out the same experiments by employing isobutyltrimethoxylsilane (BTMS) in place of MPM. BTMS is close to MPM in size and polarity, but does not contain a polymerizable vinyl group. Synthesis of BTMS–MPS-capped QDs was carried out successfully by employing the same procedure that gave the MPM–MPS-capped QDs.
Synthesis of CdSe@ZnS QD-PS composite beads
Typically, 1 g of polyvinyl alcohol (PVA) (87–89% hydrolyzed PVA, average MW 85,000–146,000) was dissolved in 100 mL of water and degassed with N2 for 20 min. A monomer mixture was prepared by dissolving 0.1 g of MPM–MPS-capped CdSe@ZnS QDs, 0.5 mL of divinylbenzene and 0.1 g of 2,2′-azo-bis-isobutyronitrile (AIBN) in 10 mL of styrene. The monomer solution was then added to PVA solution with rapid stirring. The suspension was heated to 70 °C in 30 min and kept for 10 h while stirring. The as-synthesized QD-PS composite beads were obtained after washing with methanol, methanol/tetrahydrofuran (THF) (1/1, v/v), THF, CH2Cl2, and acetone in that order.
Optical Characterization
FT-IR spectra were taken by employing Nicolet 6700 from Thermo Fisher Scientific Inc. The UV–Vis and PL spectra were measured with Shimadzu UV-2100U spectrophotometer and Jobin Yvon Fluoromax-3 Spectrofluorometer, respectively. A photobleaching experiment was performed to study the photostability of QD-PS microbeads by continuously illuminating the microbeads in a Starna semi-micro fluorometer cell (Type 9F) using femtosecond Ti:Sapphire laser system with a laser beam at 532 nm wavelength and power density of 1.4 W/cm2. To prevent the QD-PS microbeads from precipitation, they were suspended in Tween 20 aqueous solution. The PL intensity was recorded every 10 min.
Photoluminescence quantum yield was determined by a comparative method, which involves the use of a standard solution of known quantum yield [14]. The PL quantum yield of the QD solutions was determined using rhodamine B in ethanol as standard solution. A ratio of the integrated fluorescence intensities of the sample and standard solutions was used to calculate the QY according to the following equation [15]:
where the subscripts S and R denote sample and rhodamine, respectively, QY the fluorescence quantum yield, A the absorbance, I the maximum intensity of the emission peak, and n the refractive index of the solvent. The fluorescence yield of rhodamine B is reported to be 49% in ethanol [16].
Results and discussions
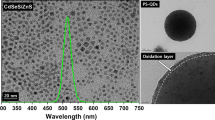
The three different oleylamine-capped CdSe QD products showed PL with λmax = 540, 584, and 614 with a quantum yield of 25, 15, and 3.3%, respectively. The X-ray diffraction patterns of all the samples exhibited a zinc-blende type structure, as reported early. Figure 1 shows the FT-IR spectra of the four samples from each step: (a) oleylamine-capped CdSe QDs, (b) MPS-capped CdSe@ZnS QDs, (c) MPM–MPS-capped CdSe@ZnS QDs, and (d) QD-PS microbeads. The spectra (b) and (c) clearly exhibit a peak at 2,562 cm−1 that corresponds to the S–H stretching mode, indicating the presence of the MPS molecules in the products [17]. The same peak does not appear in the spectrum (d) because the peaks from PS dominate the spectrum. The peak at 1,718 cm−1 in the spectrum (c) corresponds to the C=O stretching mode and hence confirms the coupling between the MPS and MPM molecules.
However, a more practical indicator of the successful ligand exchange and coupling is the change in solubility of the QDs in various solvents. Namely, we have observed that MPS-capped QDs are readily soluble in methanol while the oleylamine-capped QDs dissolve well in hexanes [11]. This solubility change is in fact utilized to purify the MPS-capped QDs after the ligand-exchange reaction. Likewise, the subsequent coupling of MPM to MPS molecules on the QDs makes the QD not soluble in methanol. Therefore, the MPM–MPS-capped QDs precipitate out from the reaction solution. The MPM–MPS-capped QDs were readily soluble in styrene.
The successful ligand exchange was also confirmed by the enhancement of PL intensity (e.g., the quantum yields of the MPS-capped QDs were 78, 65, and 16% for the green-, orange-, and red-emitting CdSe@ZnS QDs, respectively, which are much higher than those of the original oleylamine-capped CdSe QDs (25, 15, and 3.3%)). Figure 2a shows the PL from MPS-capped CdSe@ZnS QDs (dashed), MPM–MPS-capped CdSe@ZnS QDs (dotted), and CdSe@ZnS QD-PS microbeads (solid), from three different sizes of the QDs. The full-width-half-maximum (FWHM) values of the emission peaks are in the range of 50–60 nm. Importantly, the peak shapes did not change upon the reactions and the quantum yields were 75, 61, and 14% for green-, orange-, and red-emitting QDs, respectively, after the MPM coupling process. The obtained QD-PS hybrid products showed bright emission that was homogenous among the microbeads (see below). The well-shaped PL spectra of the polystyrene microbeads (Fig. 2a) support the successful incorporation of the QDs in the polystyrene and the absence of other optical phenomena. The quantum yield was not measured because of the problem of the concurrent excitation of the polymer matrix. A small red shift in the PL peak position was observed in the QD-PS microbeads compared with the MPS-capped QDs and MPM–MPS-capped QDs, which is consistent with previous reports [4, 18, 19]. This is due to the increase in the refractive index of the surrounding optical medium. The additional broad shorter-wavelength emission in the spectra of CdSe@ZnS QD-PS microbeads can be attributed to the polystyrene matrix. Figure 2b shows the change in quantum yield of the QD-PS microbeads, while they were exposed continuously to an intense laser beam. The PL intensity decreases to 80% after 1 h and the decrease slows down significantly afterward.
When the same polymerization reaction was carried out with BTMS–MPS-capped CdSe@ZnS QDs, it was found that the precipitate product showed a white color without any PL while the supernatant solution exhibited the PL, indicating the unsuccessful incorporation of the QDs in the PS microbeads. As mentioned earlier, BTMS is similar to MPM in size and polarity but does not contain a polymerizable functional group. Therefore, it confirms that incorporation of the QDs is not possible without a polymerizable functional group in our reaction process.
Figure 3 shows the scanning electron microscopy (SEM, XL30 ESEM-FEG, Philips) photographs and the pseudo-color images under a confocal microscope (LSM5, Carl Zeiss) of the as-synthesized CdSe@ZnS QD-PS microbeads. Various sizes of the microbeads were obtained from different reaction conditions. The brightest microbeads were obtained for the green-emitting QDs, which had the highest quantum yield. The strong and relatively homogenous PL of the QD-PS microbeads indicates that the QDs are well incorporated in the polymer matrix.
Conclusions
We have developed a simple method for preparation of highly photoluminescent and photostable QD-polymer microbeads, by employing a straightforward functionalization of the QD surface with polymerizable molecules through concomitant ZnS shell formation and ligand exchange, followed by an organosilane coupling process. The results indicate that the QDs maintain the same optical characteristic over our synthetic process and that the products are relatively stable even under continuous exposure to an intense laser beam. By taking advantage of the well-established organosilane coupling chemistry with an extensive list of functionalized organosilane coupling agents that are readily available [20], the strategy reported here should be widely applicable to QD surface functionalization and fabrication of QD-based composites.
References
Han M, Gao X, Su JZ, Nie S (2001) Nat Biotechnol 19:631
Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A (2002) Science 298:1759
Pelligrino T, Manna L, Kudera S, Liedl T, Koktysh D, Rogach AL, Keller S, Rädler J, Natile G, Park WJ (2004) Nano Lett 4:703
Li Y, Liu CY, Pickett N, Skaba PJ, Cummins SS, Ryley S, Sutherland AJ, O’Brien P (2005) J Mater Chem 15:1238
Allen CN, Lequex N, Chassenieux C, Tessier G, Dubertret B (2007) Adv Mater 19:4420
Sheng W, Kim S, Lee J, Kim S-W, Jensen K, Bawendi MG (2006) Langmuir 22:3782
Petruska MA, Malko AV, Voyles PM, Klimov VI (2003) Adv Mater 15:610
Kalyuzhny G, Murrary RW (2005) J Phys Chem B 109:7012
Gerion D, Pinaud F, Williams SC, Parak WJ, Zanchet D, Weiss S, Alivisatos AP (2001) J Phys Chem B 105:8861
Wang Q, Iancu N, Seo D-K (2005) Chem Mater 17:4762
Iancu N, Sharma R, Seo D-K (2004) Chem Comm 2298
Wang Q, Seo D-K (2006) Chem Mater 18:5764
Wang Q, Xu Y, Zhao X, Chang Y, Liu Y, Jiang L, Sharma J, Seo D-K, Yan H (2007) J Am Chem Soc 129:6380
Williams ATR, Winfield SA, Miller JN (1983) Analyst 108:1067
Demas JN, Crosby GA (1971) J Phys Chem 75:991
Kubin RF, Fletcher AN (1982) J Luminescence 27:455
Socrates G (1980) Infrared characteristic group frequencies. Wiley, New York
Skaff H, Sill K, Emrick T (2004) J Am Chem Soc 126:11322
Lee J, Sundar V, Heine J, Bawendi MG, Jemsen KF (2000) Adv Mater 12:1102
Plueddemann EP (1982) Silane coupling agents. Plenum Press, New York
Acknowledgement
D.-K.·S. is grateful for financial support from the National Science Foundation through his CAREER Award (DMR Contract No. 0239837) and the Camille and Henry Dreyfus Foundation for his Camille Dreyfus Teacher-Scholar Award. The authors thank Dr. Douglas Daniel for his help in confocal microscopy studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Seo, DK. Preparation of photostable quantum dot-polystyrene microbeads through covalent organosilane coupling of CdSe@Zns quantum dots. J Mater Sci 44, 816–820 (2009). https://doi.org/10.1007/s10853-008-3138-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-3138-4