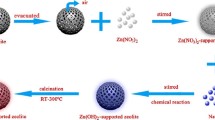
Abstract
Silver ion was loaded into zeolite A by a rapid synthesis method, microwave loading, to obtain an antibacterial agent suitable for use in biological applications. Antibacterial activity of silver-loaded zeolite A against Escherichia coli, Bacillus subtilis, and Staphylococcus aureus was determined by minimum inhibitory concentration, revealing its potential as a strong bactericide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human beings are under the threat of microbes in their living environment every day. Effective antibacterial materials have attracted great attention from scientists in recent decades. Inorganic materials, which can resist heat, radiation, and mechanical stress, have become important antibacterial agents for use in biomedical [1, 2] and biological [3] applications.
Previous studies revealed that synthetic or natural zeolites (A, X, Y, and Z) and clinoptilolite assembled with inorganic metal ions such as silver, copper, etc. can be used as bactericides for water disinfection [4, 5]. Zeolites are crystalline aluminosilicates, the basic crystalline skeletons of which are composed of SiO 4−4 and AlO 5−4 tetrahedra. Cation exchange is one of the outstanding properties of zeolites. Tetrahedra constitute the typical zeolite structure, with a system of cages and channels of formula: Mx[(AlO2)x(SiO2)y] · n(H2O), where x and y represent the total number of tetrahedra in a basic cell [6]. Since the aluminum tetrahedron carries one negative charge, this must be compensated for by the M+ cation, which is not bound by a covalent bond and can therefore be very easily replaced by other elements [7].
Silver and silver ions have long been known to have powerful antibacterial capability and low toxicity [8]. Investigation of the inhibition mechanism of Ag+ on microorganism indicated that it affects DNA molecules, disabling their replication abilities, and interacts with thiol groups in protein, inducing inactivation of bacterial proteins [9, 10]. Due to its chemical as well as physical durability [11] and ability to release Ag+ for a long period of time [12], silver-loaded zeolite also proved to be a useful vehicle with strong antibacterial activity, as used in dental materials [13]. Moreover, silver-loaded products, such as Zeomic, have been used as sterilizing and antibacterial agents in paper, plastic, paint, ceramics, and almost everything with which we come into contact in our daily lives [14–16]. Conventional heating is the traditional method to obtain these kinds of material [17, 18]. Following the development of microwave techniques, microwave heating has found a number of applications in synthetic chemistry [19]. Compared with conventional heating it has a number of advantages such as short reaction time, improved product uniformity, and high purity [20–22]. The actual mechanism by which microwaves influence zeolite synthesis is not understood, and various hypotheses and explanations have been proposed [18]. In our previous studied, rare-earth-doped (including silver-doped) spherical phosphor materials with nanometer sizes [23, 24], mesoporous molecular sieve MCM-41 [25], and zeolite [26] have been synthesized successfully by microwave technique.
Here we report a fast preparation method for silver-loaded zeolite A (AgZm) by microwave-loading method. The formation of antibacterial AgZm was confirmed by energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), infrared (IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), ultraviolet–visual (UV–Vis) spectroscopy, scanning electron microscopy (SEM), and differential thermal analysis (DTA). Antibacterial activities against Gram-negative Escherichia coli (E. coli, ATCC 10231) and Bacillus subtilis (B. subtilis, ATCC 6633), and Gram-positive Staphylococcus aureus (S. aureus, ATCC 27154), were also investigated. Results suggested that these high-quality nanometer-size materials could be easily obtained and scaled up for industrial applications because of their antibacterial activity.
Experimental method
Materials
Sodium-type zeolite A was supplied by Shandong Aluminium Corporation (SALCO, China) with Ca exchange capability ≥310 (CaCO3 mg/z-g). AgNO3 and other reagents, of a commercial analytical grade (produced by Guangdong Guan Hua Chemical Factory CO., LTD, China), were used without further purification.
S. aureus (ATCC 27154), B. subtilis (ATCC 6633), and E. coli (ATCC 25922) used in minimum inhibitory concentration (MIC) tests were provided by College of Life Science, Sun Yat-sen University.
Preparation of the AgZ sample by microwave-loading method
Five grams zeolite powder was treated with 100 mL 0.1 mol/L AgNO3 solution, adjusted the solution pH value to 2 to 8. The mixture was placed in a domestic microwave oven and heated for about 15–30 min. The reaction mixture was left overnight to improve absorption of Ag+. The reacted mixture was separated by centrifugation. The resulting solid was washed with deionized water until Ag+ testing with dilute HCl was negative, and then dried at 80 °C for 4–5 h to obtain AgZm nanoparticles.
Preparation of the AgZ sample by conventional heating method
Five grams zeolite powder was treated with 100 mL 0.1 mol/L AgNO3 solution, adjusting the solution to pH 4.7. The mixture was treated in a 85 °C water bath, heated by an electronic oven, with magnetic stirring for 30 min and left overnight. The reacted mixture was separated by centrifugation. The solid phase was washed with deionized water until Ag+ testing with dilute HCl was negative, and then dried at 80 °C for 4–5 h to obtain the AgZc sample.
Antibacterial activity experiment
Antibacterial activity of AgZc and AgZm samples was evaluated by determination of minimal inhibitory concentrations (MIC) following the broth dilution method. A dilution of AgZm or AgZc samples was prepared freshly for each experiment. Sample was suspended in 9 mL LB broth at concentration of 800 μg/mL and twofold serially diluted (400, 200, 100, 50, and 25 μg/mL) with broth. Zeolite A was suspended at concentration of 800 μg/mL and diluted in the same manner as controls.
Each strain of microorganism was preculture in LB broth aerobically at 37 °C for 12 h. Cultures were centrifuged twice (10,000 rpm), and cells were washed and suspended in distilled water, reaching a final concentration of approximately 106 cells/mL. Then, 0.5-mL aliquots of inocula were added to 4.5 mL of a series of sample and control dilution broths. The bacteria were cultured at 37 °C with gentle stirring to prevent samples from sedimenting and to ensure they came into contact with the test agents. The magnetic stirrer was stopped 1 h before measuring MIC.
After 24 h of incubation, 50 μL was taken from the above mixture (broth + bacteria + samples). These aliquots were spread on LB agar plates and incubated at 37 °C for 24 h. Viable bacterial colonies were counted and recorded by naked eye, determining the lowest concentration that lacked bacteria growth as the MIC. Tests were performed three times for each strain to calculate the average value as the final MIC value.
Apparatus
Microwave radiation apparatus was a commercial domestic microwave oven model WD800 with frequency 2,450 MHz and power 800 W (LG, Korea). Microanalyses (energy dispersive spectroscopy, EDS) were carried out by using a model S-520/INCA 300 EDS (Oxford, Japan). XRD data were measured by D/MAX2200 diffractometer (Rigaku, Japan) coupled to a copper-anode X-ray tube. IR spectra in the frequency range 400–4,000 cm−1 were determined at room temperature by Fourier-transform infrared (FT-IR) VECTORWW spectrometer (Bruker, Germany) as KBr discs (sample/KBr mass ratio 1:100). XPS was carried out with an Axis Ultra instrument (Kratos, England). UV–Vis absorption spectra were carried out with UV-3100 spectrophotometer (Shimadzu, Japan). SEM surface morphology and the mean grain sizes of the samples were observed with XL-30 SEM (Philips, The Netherlands) operating at 20 kV. DTA was obtained by Differential thermal analyzer (Perkin-Elmer, USA).
Results and discussion
Optimum reaction condition for AgZm synthesis was determined by reacting under various conditions (Table 1). Data showed that ion exchange resulted in maximum Ag+ absorption (100%) when mixture pH was adjusted to 4.7 and reacted under 360 W microwave power for 15 min. The ion exchange reactions were incomplete when the power was less than 360 W; and the crystalline structures of zeolite A were destroyed when microwave power was more than 480 W. It was also found that the crystalline structures were destroyed under highly acidic conditions (mixture pH < 3.0). On the other hand, AgZm samples became dark when the pH value of the mixture was greater than 7.5 because Ag+ in the zeolite turned to metal silver Ag0 under weak basic conditions.
The fourth AgZm sample listed in Table 1 (ion exchange degree 100%) was used as the test sample for the followed experiments. EDS patterns of zeolite A before and after loading with Ag+ are shown in Fig. 1, and relevant elemental composition of samples are listed in Table 2. As can be seen in Fig. 1a, three peaks representing Na, Al, and Si, respectively, can be observed. This indicates that zeolite A possessed the typical sodium-type zeolite A crystal structure and the normal chemical composition [27]. After the Ag+ exchange reaction, the Na peak disappeared and Ag peaks were present (Fig. 1b). It can be concluded that Na+ was exchanged by Ag+ [4]. Atomic percentages (Table 2) also support this deduction: atomic percentage of Na+ decreased from 11.45% to 0%, while Ag+ increased from 0% to 16.16%.
To compare the quality of samples obtained by the conventional (AgZc) and the microwave-loading method (AgZm), properties testing was carried out for each sample. XRD patterns of the starting zeolite A, AgZc, and AgZm are shown in Fig. 2. Curve 1 represents the characteristic peaks of the crystalline structure of zeolite A, in agreement with the reference value in JCPDS39-0222. The peak positions of AgZc (curve 2) and AgZm (curve 3) were almost the same as those of zeolite A, with minor changes in their intensity. This indicates that the samples essentially maintained the crystal configuration of the starting zeolite A but, following the replacement of Na+ with Ag+, the electronic field in the crystalline zeolite changed and led to changes in the relative intensity.
IR spectra of AgZm and AgZc samples are presented in Fig. 3. The five characteristic peaks of zeolite A, located at 3,440, 1,008, 667, 560, and 468 cm−1 due to the vibration bands of H2O, SiO 4−4 , and AlO 5−4 tetraeders bending mode, were observed in both samples [28–30]. This also proves that samples maintained the zeolite A structure. The vibration bands of NO −3 (1,350–1,400 cm−1) were not observed. It is proposed that Ag+ entered the zeolite network, occupying ion exchange sites, or existed as other silver composites [5, 31].
Optical UV–Vis absorption spectra of AgZm and AgZc samples are shown in Fig. 4. It can be seen that both samples have a broad but not sharp peak within the range 200–400 nm. This may because the Ag+ coordinated with the O2− structure of zeolite A, and the Ag+ in the zeolite possibly exits as other oxide composites.
XPS patterns of AgZm and AgZc samples are shown in Fig. 5. It can be seen that the two samples had the same binding energy of 368.2 eV. The results of XPS analysis are fully consistent with those reported previously [32], indicating that silver in the samples was in an ionic state.
SEM photographs of zeolite A, AgZc, and AgZm samples are shown in Fig. 6. Compared with zeolite A, it was easily observed that there were particles in a size range of 10–50 nm on the surface of AgZc and AgZm. Fewer particles were located on the surface of AgZc than on AgZm. It was supposed that the rate of exchange with microwave loading was much faster than with conventional heating, so the pores of the zeolite A were rapidly jammed and subsequent silver ions aggregated on the surface of the samples.
Differential thermal analysis (DTA) patterns are shown in Fig. 7. Differences between the DTA data for the samples can be clearly seen. The dehydration temperatures of starting zeolite A, AgZc, and AgZm were 190 °C, 165 °C, and 153 °C, and the decomposition temperature were 900 °C, 753 °C, and 756 °C, respectively. There was slight difference between the AgZc and AgZm in terms of both dehydration and decomposition temperature. Moreover, there was a strong exothermic peak at 465 °C in the curve of AgZm, accounted for by the decomposition of silver oxide [10]. Compared with the starting zeolite A, both the dehydration temperature and the decomposition temperature of AgZc and AgZm were lower.
Antibacterial testing for antibacterial activity revealed that AgZm samples acted as excellent antibacterial agents against both Gram-positive and Gram-negative bacteria. Table 3 shows antibacterial activity against E. coli, B. subtilis, and S. aureus microorganisms. The MIC value of the AgZm sample was 50 μg/mL, while that of the AgZc sample was 100 μg/mL, indicating that the former has better antibacterial ability (Table 3).
Conclusions
Silver-loaded zeolite nanoparticles were obtained using a fast low-cost microwave-loading method. These nanoparticles have better quality than those obtained by conventional method. It was proved that silver-loaded zeolite nanoparticles have significant antibacterial ability.
According to the results, we conclude that silver-loaded zeolite synthesized by the microwave-loading method can be used as an antibacterial agent in wide applications.
References
Tartaj P, del Puerto Morales M, Veintemillas-Verdaguer S, González-Carreño T, Serna CJ (2003) J Phys D Appl Phys 36:R182
Hütten A, Sudfeld D, Ennen I, Reiss G, Hachmann W et al (2004) J Biotech 112:47
Sondi I, Salopek-Sondi B (2004) J Colloid Interface Sci 275:177
Rivera-Garza M, Olguín MT, García-Sosa I, Alcántara D, Rodríguez-Fuentes G (2000) Microporous Mesoporous Mater 39:431
Top Ayben, Ulku Semra (2004) Appl Clay Sci 27:13
Čík G, Bujdáková H, Šeršeň F (2001) Chemosphere 44:313
McCann GF, Millar GJ, Browmaker GA, Coonney RP (1995) J Chem Soc Faraday Trans 91:4321
Jeon HJ, Yi SC, Oh SG (2003) Biomaterials 24:4921
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) J Biomed Mater Res 52:662
Kawashita M, Tsuneyama S, Miyaji F, Kokubo T, Kozuka H, Yamamoto K (2000) Biomaterials 21:393
Xu BS, Hou WS, Wang SH et al (2008) J Biomed Mater Res B 84B:394
Toshikazu T (1999) Inorg Mater 6:605
Langella A, Pansini M, Cappelletti P, de Gennaro B, de’ Gennaro M, Colella C (2000) Microporous Mesoporous Mater 37:337
Liang J, Jin Z, Wang J (1999) J Chin Ceram Soc 27:16
Niira R, Yamamoto T, Uchida M (1990) US Patent 4938958
Takai K, Senda Y et al (2002) Microbiol Immunol 46(2):75
Viertelhaus M, Taylor AE, Kloo L, Gameson I, Anderson PA (2006) Dalton Trans 2368
Onodera Y, Iwasaki T, Chatterjee A, Ebina T et al (2001) Appl Clay Sci 18(3–4):123
Adam D (2003) Nature 421(6923):571
Cundy CS (1998) Collect Czech Chem Commun 63:1699
Tompsett GA, Conner WC, Yngvesson KS (2006) Chem Phys Chem 7:296
Panzarella B, Tompsett GA, Yngvesson KS, Conner WC (2007) J Phys Chem B 111:12657
Zhang M (2000) Rare Met 19:279
Li J, Zhang M, Yan C (2003) Acta Opt Sin 23:604
Zhang M, Li L, Yang Y (1998) Acta Univ Sun Yatseni (Scientiarum Naturalium) 37:128
Zhong S, Zhang M, Su Q (2005) Chin J Appl Chem 22:865
Pilter Z, Szabó S, Hasznos-Nezdei M, Pallai-Varsányi E (2000) Microporous Mesoporous Mater 40:257
Chu P, Dwyer FG, Vartuli VC (1998) US Patent 4 778 666; European Patent Appl 0 358 827 (1990)
Xu R, Pang W, Tu K (1987) Zeolite molecular sieves structure and synthesis. Chuangchun, China
Stojkovic SR, B Adnadjevic (1988) Zeolites 8:523
Zinner LB, Araújo AS (1992) J Alloys Compd 180:289
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1995) Handbook of X-ray photoelectron spectroscopy Perkin–Elmer. Eden Prairie, Minnesota
Acknowledgement
This work is supported by the National Natural Science Foundation of China (20772162).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhong, S., Zhang, M. et al. Antibacterial activity of silver-loaded zeolite A prepared by a fast microwave-loading method. J Mater Sci 44, 457–462 (2009). https://doi.org/10.1007/s10853-008-3129-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-3129-5