Abstract
Simple routes to synthesize highly photoluminescent monodisperse thiol-capped CdSe nanoparticles are reported. The as-synthesized water-soluble CdSe nanoparticles possess higher photoluminescence quantum efficiency (up to 22%), smaller values of full width of half-maximum of photoluminescence spectra (down to 31 nm), higher photostability, and better monodispersity compared with the nanoparticles made from other aqueous routes. The quality of CdSe nanoparticles prepared by our route compares very favourably with those prepared in organic solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is currently intense interest in making II–VI colloidal semiconductor nanoparticles (NPs) for different applications [1–9]. Among the various kinds of semiconductor NPs, colloidal CdSe is the most widely investigated because their emission can be easily tuned to cover from red (centred at 650 nm) to blue (centred at 450 nm) as the size of NPs decreases [10]. CdSe NPs can be prepared in either aqueous or organic solutions. The properties of as-prepared CdSe NPs by different methods are summarized in Table 1.
From the table it can be seen that TOPO(trioctylphosphine oxide)-TOP (trioctylphosphine) route produces CdSe NPs with very good properties, such as high photoluminescence (PL) quantum efficiency (QE), narrow full width of half-maximum (FWHM) of PL spectra, excellent monodispersity and high photostability. It has thus been one of the most successful methods to synthesize CdSe NPs. However, these CdSe NPs are only soluble in some nonpolar organic solvent, which raises problems if they are to be used for biological applications. Even though surface modification can be applied to make semiconductor NPs water-soluble, such as CdSe/ZnS [2, 11], CdSe(CdS) [1] or CdTe [12], the methods involve several extra procedures and often produce water-soluble NPs with a lower QE [1, 12]. In the case of CdSe NPs, the emission has been observed completely lost after being transferred into water, if no wider band gap shell is used [12]. Up to now, there has been no report of any very successful aqueous route for preparing CdSe NPs of quality comparable with the NPs produced by the TOPO-TOP route. In this article, we describe a simple synthetic route of preparing high-quality CdSe NPs in aqueous solution. This has been achieved by the reaction of cadmium acetate and sodium selenosulphate in the presence of thioacids. We admit that similar approaches have been used in CdSe NP synthesis [13]. However, the synthesis conditions used in literature are far worse than optimal while the conditions are of great importance for making high-quality CdSe NPs.
Experimental
Cadmium acetate, sodium sulphite, sodium hydroxide, selenium powder, 3-mercaptopropionic acid (3-MPA), and mercaptosuccinic acid (2-MSA) were obtained from Sigma–Aldrich. A solution of 0.02 M sodium selenosulphate was formed by refluxing 0.5 g sodium sulphite and 0.05 g selenium powders in 33 mL water at 80 °C for 5 h. The prepared colourless sodium selenosulphate solution was stored under dry nitrogen for later use.
Synthesis procedures
A solution of 0.064 g (0.24 mmol) of Cd(Ac)2 · 2H2O and 50.2 μL of 3-MPA (or 0.086 g of 2-MSA) (0.58 mmol) in 48 mL of demineralized water was adjusted to pH 9.3 with 1 M NaOH solution. The solution was clear and colourless. It was placed in a three-necked flask fitted with a septum and valves and was de-aereated by bubbling with N2 for 1 h. Under vigorous stirring, different amounts of the stored oxygen-free 0.02 M sodium selenosulphate solution were injected. The colour of the solution turned to bright-green after a few minutes refluxing. The temperature of the reaction system was maintained at 100 °C.
Photoactivation of CdSe nanoparticles
The photoactivation of NPs was carried out in an ambient atmosphere (1 atm, 23 °C). The NPs solution contained in a glass beaker was exposed to ambient sun light on daytime and room light during night. The beaker was covered by parafilm with small holes.
Characterization of CdSe nanoparticles
The UV–Vis absorption and PL of the as-prepared CdSe NPs were recorded using a double-beam UV–Vis spectrometer (Perkin-Elmer Lambda 19) and a PL spectrometer (Hitachi, FL-4500), respectively. All absorption and PL spectra were measured without any post-preparative size separation. The QE of 2-MSA-CdSe NPs was determined by comparison with the value of the QE of Rhodamine 6G in ethanol at concentration of ~10−7 M (λex = 480 nm, QE = 95%). The QE of 3-MPA-CdSe NPs was compared with the value of the QE of Rhodamine 6G in ethanol at concentration of ~10−6 M (λex = 400 nm, QE = 80–85%). (Because the PL peak of 3-MPA-CdSe NPs starts from about 420 nm and the absorption of ~10−7 M Rhodamine 6G at around this wavelength is too low to be measured accurately.) The following procedures were used to measure the PL QE of CdSe NPs [19]. Briefly, the absorbance at the excitation wavelengths and the fluorescence spectra for the Rhodamine 6G and CdSe NP samples were measured. Six different concentration solutions were used for both the Rhodamine 6G and CdSe NP sample. The absorbance at excitation wavelength was below 0.1 for all samples. The integrated fluorescence intensity (i.e., the area of the fluorescence spectrum) from the PL spectrum was calculated for each sample. Next, a graph of integrated fluorescence intensity versus absorbance at excitation wavelength was plotted. The result was a straight line with gradient m, and intercept 0 for both the Rhodamine 6G and CdSe NP samples. Finally, the following equation was used to calculate the PL QE of CdSe NP samples:
where Φ is the quantum efficiency, Grad the gradient from the plot of integrated fluorescence intensity versus absorbance, and η the refractive index of the solvent (ηwater ≈ 1.335, ηethanol ≈ 1.362).
The size distribution and crystalline structure of the as-prepared CdSe NPs were determined by high-resolution transmission electron microscopy (HRTEM). The NPs were supported on an amorphous carbon film and the images recorded at 400 kV in a JEOL 4000EX microscope. X-ray diffraction (XRD) patterns were taken by a Philips PW1710 diffractometer using Cu Kα radiation.
Results and discussion
Figures 1 and 2 present the evolution of absorption and PL spectra of 3-MPA and 2-MSA stabilized CdSe NPs, respectively, as a function of refluxing time at 100 °C. After refluxing, the colour of solution gradually changed and NPs started to form. At the beginning of the NP formation, e.g. 2-MSA-CdSe NPs after 20 min refluxing, only the surface state PL at long wavelengths can be seen in the spectrum shown in Fig. 2. The spectrum is extremely broad and the peak at ~560 nm is shifted strongly towards longer wavelength in comparison with the absorption edge at 440 nm. It is likely that there are a large number of surface defects at the early stages of NP nucleation, and the surface state density is high for small particles [20]. As the refluxing time increased, for instance for 3-MPA-CdSe NPs after 4 h refluxing, the near band-gap PL peak started to appear at ~458 nm (pure blue light) although the surface state PL feature was still dominant. After longer refluxing times, the peaks of near band-gap PL spectra of the NPs gradually red-shifted. In the meantime, the near band-gap PL of the NPs became stronger while the surface state PL greatly decreased. It can be seen from Fig. 1 that the surface trap emission almost disappears when the emission wavelength of 3-MPA-CdSe NPs reaches about 500 nm, while it remains prominent for the similar sized CdSe NPs prepared by TOPO-TOP route [20]. This suggests that the small CdSe NPs (emission wavelength less than ~540 nm) prepared by our aqueous route have better control of the surface states than those prepared by the TOPO-TOP route. The PL and UV-visible absorption spectra (Fig. 2) of our as-prepared 2-MSA-CdSe NPs are very sharp, with about 33–34 nm FWHM in the PL spectra. These as-prepared NPs showed very high near band-gap PL compared with those produced by other aqueous routes. The QE of as-prepared 3-MPA-CdSe NPs (initial Cd:Se = 10:1, after 72 h refluxing) and 2-MSA-CdSe NPs (initial Cd:Se = 10:1, after 102 h refluxing) reached 14% and 10.7%, respectively, as determined by reference to the value for Rhodamine 6G in ethanol.
Figure 3 shows the temporal evolution of PL spectra of four different reactions with different initial Cd:Se ratios. We note several important features: first, with increasing Cd:Se ratio of the precursors, the PL spectrum peaks of CdSe NPs red-shift more rapidly. It took 112 h for the reaction system with the initial Cd:Se ratio 5:1 to prepare CdSe NPs whose emission wavelength is about 520 nm, against only 16 h for that with an initial Cd:Se ratio 30:1. Second, the PL QY of the as-prepared CdSe NPs increased monotonically to a maximum and then gradually decreased. Third, the reaction system with the initial Cd:Se ratio of 10:1 or 15:1 prepared the most highly luminescent CdSe NPs. Either increasing or decreasing the initial Cd:Se ratio will lead to a lower PL QE of as-synthesized CdSe NPs for the following reason. According to Peng et al. [10], if one precursor was initially in large excess, the concentration of that monomer in solution may be considered to be constant at the beginning stage, so it would provide a favourable condition for the construction of the most favourable surface structure for the CdSe NPs in the solution. Correspondingly, the PL QE of the as-prepared CdSe might be higher. Our HRTEM results showed that the CdSe NPs prepared from the reaction system with the initial Cd:Se ratio of 10:1, 15:1, and 30:1 had much better monodispersity than those prepared from the reaction system with the initial Cd:Se of 5:1. Another reason for the PL QE of CdSe NPs changing with the initial CdSe ratios is explained as the following. The temperature-dependent extended X-ray absorption fine structure (EXAFS) study on 2-mercaptoethanol capped CdTe NPs had suggested that the extremely small nanoparticles were consisted of a CdTe zinc blende core partially coated by a Cd-SR (R = organic rest in thiols) [21]. A similar structure was also observed in NPs when other thiols were used, such as, mercaptoacids, mercaptoalcohols, dithiols. The reason was the partial hydrolysis of thiols caused an incorporation of the sulphur from the thiol molecules into the growing NPs under prolonged refluxing. The deviations of the XRD reflexes from zinc blende diffraction patterns of NPs and bulk indicated the intensity of the sulphur incorporation from the thiol molecules into the NPs [22, 23]. In our experiments, XRD results showed that the diffraction peaks for 3-MPA stabilized CdSe NPs significantly shifted to high angles when the initial Cd:Se ratio increased from 10:1 to 30:1 while the other conditions were same [24]. It indicated that a large amount of sulphur was incorporated into CdSe NPs when the initial Cd:Se ratio reached a very high value of 30:1. The initial sulphur incorporation in NPs can form a CdS shell structure on CdSe NPs, which aids the confinement of the exciton on the CdSe core and, therefore, greatly enhances the PL QE of CdSe NPs [25, 26]. However, as the thickness of the CdS shell continually increases, high concentration of structural defects will be created within the thick deposited shell because of the mismatch between the crystalline lattices of CdSe core and CdS shell [27, 28]. Therefore, the PL QE of CdSe NPs deteriorates when the initial Cd:Se ratio reached up to 30:1. From Fig. 3d it can be seen that the surface trap emission of CdSe NPs prepared from the reaction system with the initial Cd:Se ratio of 30:1 is more prominent than those prepared from the reaction system with smaller initial Cd:Se ratio. Fourth, with increasing reaction time, the PL FWHM of 3-MPA-CdSe NPs continually decreased or remained almost constant after the PL QE of CdSe NPs reached the PL maximum point, which is different from the observation by Peng et al. [10] who found that the PL FWHM normally started to increase after the PL reached maximum point.
Similar investigations have also been done on 2-MSA-capped CdSe NPs and the results are shown in Fig. 4. There are several features that are different from those of 3-MPA-CdSe NPs. First, with increasing initial Cd:Se ratio of the precursors, the PL spectrum peaks of CdSe NPs do not red-shift much. After 80 h refluxing, the reaction system with the initial Cd:Se ratio of 10:1 produced CdSe NPs whose emission wavelength is about 553 nm, against 561 and 564 nm for those with initial Cd:Se ratios 20:1 and 30:1, respectively. Second, the absorption peaks of CdSe NPs prepared from the system with initial Cd:Se ratio 30:1 became extremely sharp (Fig. 4a) and the FWHM of PL spectra became very narrow, down to about 31 nm, an indication of good quality and narrow size distribution of the NPs. It is rarely seen for the NPs prepared from aqueous routes in literature [13, 15–17]. Third, the highest PL QE of the as-prepared CdSe NPs increased monotonically with increasing initial Cd:Se ratio. The highest PL QE reached 22% for CdSe NPs prepared by the reaction system with initial Cd:Se = 30:1.
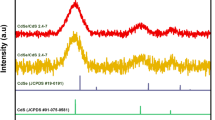
The XRD patterns for 3-MPA-CdSe NPs (initial Cd:Se = 15:1, refluxing at 100 °C for 62 h) and 2-MSA-CdSe NPs (initial Cd:Se = 30:1, refluxing at 100 °C for 82 h) are shown in Fig. 5. The broad peaks imply that the CdSe NPs are very small. The patterns also confirm the crystalline cubic structure of CdSe NPs. Diffraction peaks shown in this pattern correspond to the (111), (220), and (311) plane reflections of cubic CdSe. The diffraction peaks shown in the figure are intermediate between the values of the cubic CdSe and the cubic CdS phases. The reason is the partial hydrolysis of mercapto acids caused an incorporation of the sulphur from the mercapto acids molecules into the growing NPs under prolonged refluxing [22, 23]. It can also be seen from Fig. 5 that the diffraction peaks of 2-MSA and 3-MPA stabilized CdSe NPs are at about same positions even though 3-MPA-CdSe NPs were prepared at much smaller initial Cd:Se ratio and shorter refluxing time. Because of this, higher initial Cd:Se ratio can be adopted to prepare CdSe NPs with higher near band-gap PL when 2-MSA is used as stabilizer.
XRD patterns of 3-MPA-CdSe (top) and 2-MSA-CdSe (bottom) nanoparticles. Also included are the standard diffraction lines of bulk CdSe and CdS with cubic structure provided by the JCPDS database. The peaks at 2θ of 25.4, 42.0, and 49.7° correspond to (111), (220), and (311) planes of cubic CdSe, respectively. The peaks at 2θ of 26.6, 30.8, 44.0, and 52.1° correspond to (111), (200), (220), and (311) planes of cubic CdS, respectively
Figure 6 shows an HRTEM image of 3-MPA-CdSe NPs. It can be seen that the size of these NPs is around 3.3 nm with excellent monodispersity, suggesting that long refluxing times need not always lead to polydisperse NPs. The main lattice fringe spacings of most NPs shown in the figure are about 3.4 Å, which corresponds to the d111 spacing of 3.49 Å for the cubic (zinc blende) form. This result is consistent with XRD data shown in Fig. 5.
Figure 7 shows the absorption and PL spectra of CdSe NPs after different irradiation times in air. The optical densities of all samples increased slightly during the illumination, probably due to the evaporation of water. The PL intensity grew by about 30% during the first 6 days and then slightly dropped, then remained almost constant from 6th day to 15th day. Figure 7c shows the quantum efficiency of 3-MPA-CdSe NPs measured after different irradiation times in air. It can be seen that the PL QE of 3-MPA-CdSe NPs increased slightly (about 30%) at the beginning of illumination and then started to slowly drop. During 15 days illumination of 3-MPA-CdSe NPs solution, the peak positions of the absorption and PL spectra of 3-MPA-CdSe NPs remain nearly at same wavelength for all samples. In comparison, the peak of the PL spectra of citrate-stabilized CdSe NPs (initial Cd:Se = 16:1 and 8:1) shows a blue-shift of 14 and 24 nm, respectively, under similar conditions [16]. The PL QE of citrate-CdSe NPs can be enhanced to similar level with that of 3-MPA-CdSe NPs. However, the citrate-CdSe NPs start to precipitate from solution immediately after their PL QE reaches the maximum. The reason for this is probably that citrate-CdSe NPs are stabilized by an electrical double layer (composed of bulky citrate ions, chloride ions and the cations attracted to them) that is a rather loose shell [29] while 3-MPA-CdSe NPs are stabilized by strong covalent bonding. Therefore, the oxidation of citrate-CdSe NPs is very fast [16].
Finally, it is worth noting that CdSe NPs can be isolated from solution by addition of 2-proponal followed by centrifugation. These precipitated NPs can be dissolved in water and show the same or slightly better PL QE compared with those before purification.
Conclusions
In summary, the simple synthetic route described in this article permits the synthesis of high-quality CdSe NPs in aqueous solution. The as-synthesized thiol-capped CdSe NPs possess higher PL QE (up to 22%), smaller values of FWHM (down to 31 nm), higher photostability, and better monodispersity compared with those made from other aqueous routes. The quality of CdSe NPs prepared by our route compares very favourably with those prepared in organic solution. Compared with 3-MPA, 2-MSA provides CdSe NPs higher PL QE, narrower size distribution, smaller shift of XRD peaks to higher angles when high Cd:Se initial precursors are used.
References
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Science 281:2013
Chan WCW, Nie SM (1998) Science 281:2016
Colvin VL, Schlamp MC, Alivisatos AP (1994) Nature 370:354
Weiss EA, Chiechi RC, Geyer SM, Porter VJ, Bell DC, Bawendi MG, Whitesides GM (2008) J Am Chem Soc 130:74
Klimov VI (2007) Annu Rev Phys Chem 58:635
Chen X, Hutchison JL, Dobson PJ, Wakefield G (2008) J Colloid Interface Sci 319:140
Yin Y, Alivisatos AP (2005) Nature 437:664
Erwin SC, Zu LJ, Haftel MI, Efros AL, Kennedy TA, Norris DJ (2005) Nature 436:91
Shim M, Guyot-Sionnest P (2000) Nature 407:981
Qu L, Peng X (2001) J Am Chem Soc 124:2049
Mattoussi H, Mauro JM, Goldman ER, Anderson GP, Sundar VC, Mikulec FV, Bawendi MG (2000) Am Chem Soc 122:12142
Talapin DV, Rogach AL, Mekis I, Haubold S, Kornowski A, Haase M, Weller H (2002) Colloid Surf A 202:145
Rogach AL, Kornowski A, Gao MY, Eychmuller A, Weller H (1999) J Phys Chem 103:3065
Talapin DV, Rogach AL, Kornowski A, Haase M, Weller H (2001) Nano Lett 1:207
Rogach AL, Nagesha D, Ostrander JW, Giersig M, Kotov NA (2000) Chem Mater 12:2676
Wang Y, Tang ZY, Correa-Duarte MA, Pastoriza-Santos I, Giersig M, Kotov NA, Liz-Marzan LM (2004) J Phys Chem 108:15461
Sondi I, Siiman O, Matijevic E (2004) J Colloid Interface Sci 275:503
Lin YW, Hsieh MM, Liu CP, Chang HT (2005) Langmuir 21:728
Landes CF, Link S, Mohamed MB, Nikoobakht B, Ei-Sayed MA (2002) Pure Appl Chem 74:1675
Rockenberger J, Tröger L, Rogach AL, Tischer M, Grundmann M, Eychmüller A, Weller H (1998) J Chem Phys 108:7807
Gaponik N, Talapin DV, Rogach AL, Hoppe K, Shevchenko EV, Kornowski A, Eychmuller A, Weller H (2002) J Phys Chem B 106:7177
Rogach AL (2000) Mater Sci Eng B 69:435
Chen X (2006) Dimensionally confined semiconductors. PhD dissertation, Department of Materials, University of Oxford
Bao HB, Gong YJ, Li Z, Gao MY (2004) Chem Mater 16:3853
Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, Jensen KF, Bawendi MG (1997) J Phys Chem B 101:9463
Reiss P, Bleuse J, Pron A (2002) Nano Lett 2:781
Chen XB, Lou YB, Burda C (2004) Int J Nanotechnol 1:105
Hutter E, Fendler JH (2004) Adv Mater 16:1685
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Hutchison, J.L., Dobson, P.J. et al. Highly luminescent monodisperse CdSe nanoparticles synthesized in aqueous solution. J Mater Sci 44, 285–292 (2009). https://doi.org/10.1007/s10853-008-3055-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-3055-6