Abstract
A series of PMR-15 resin specimens were isothermally aged at 288, 316, and 343 °C over a range of time. For PMR-15 aged at 288 °C, the samples were also subjected to different aging environments including: ambient air, dry air, inert (argon), and pressurized air (0.414 MPa). Nanoindentation was performed to characterize localized mechanical properties as well as the development and growth of the oxidative layer. The measured increase in stiffness in the specimen surface oxidation layer is a manifestation of the chemical changes in the polymer occurring during oxidation. The average elastic modulus in the oxidized region is relatively insensitive to variations in aging temperature, time, and the environments. The thickness of the oxidative layer is observed to increase in the early stages of oxidation and the oxidation process eventually approaches an auto-retardation state. Aging under elevated pressure increases the thickness growth rate of the oxidation layer, while there is no significant difference in growth rate for specimens aged in dry air versus those aged in ambient air. It is shown that the measured average thickness of the oxidation layer and the transition region determined by the nanoindenter is in good agreement with optical microscopy measurements for all conditions considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
PMR-15 has been the most widely used high-temperature polyimide in the aeronautical industry for over 20 years due to its high glass transition temperature (~340 °C) and thermo-oxidative stability. It is well known that the free surfaces of high-temperature polymer matrix composites (HTPMCs) are susceptible to oxidation that leads to the development of ply cracking and accelerated degradation in the presence of thermo-mechanical loading. Ply cracking introduces new free surfaces in the composite providing pathways for oxidants that inevitably lead to degradation of the fiber matrix interfaces, reducing the life and durability of these material systems. Therefore, it is essential to fully characterize these high-temperature composites so that their physical and chemical responses from the oxidation process are fully understood. In this work, oxidative aging is described as a nonreversible, surface diffusion response in which chemical changes occur during the oxidation of a polymer. Oxidation leads to a reduction in molecular weight as a result of chemical bond breakage and weight loss due to out-gassing of low-molecular weight gaseous species.
Oxidation of PMR-15 neat resin is in general diffusion controlled such that oxygen diffuses into the surface and reacts with the polymer resulting in a surface oxidation layer. The inner core of the specimen does not oxidize but does have a bulk thermal aging response that can alter its properties. Bowles et al. [1] have conducted isothermal aging studies on PMR-15 at 204, 260, 288, and 316 °C on specimens with different geometrical shapes in order to investigate the mechanisms involved with thermal degradation. Both oxidation and dimensional changes were monitored on neat resin and composite specimens. It was found that the oxidative layers formed and progressed inward with aging time, and molecular level changes caused changes in density, material shrinkage, glass transition temperatures, dimension, dynamic shear modulus, and compression properties. It was found that the three major mechanisms of degradation in PMR-15 were an initial weight gain, a small percentage bulk material weight loss, and a large mass fraction weight loss concentrated at the surface of the polymer or composite. The surface loss predominated at temperatures higher than 260 °C. The surface loss and the bulk core loss became more equivalent below 260 °C, and between 175 and 260 °C, the initial weight change was due to a weight gain mechanism (presumably due to oxygen absorption) that diminished as the aging temperature was increased. Tsuji et al. [2] also investigated the effect of degradation on the mechanical properties of PMR-15 aged at 316 °C in air and nitrogen for durations of up to 800 h. Four-point bend tests were performed on specimens to determine the modulus of both the oxidized surface layer and the inner material. Bimaterial strip specimens consisting of oxidized surface material and unoxidized interior material were constructed and used to determine surface layer shrinkage and CTE. It was concluded that the surface layer and core material had large property differences.
Using nuclear magnetic resonance, Régnier et al. [3] studied the effect of aging on the structure and oxidation of PMR-15 resin for up to 2,000 h at 300 °C. It was found that both the fracture stress and the strain of aged resin reduced very quickly and became stable over time. They suggest that this occurred due to the growth of the oxidation layer during the first few days of aging. Thereafter, the oxidation layer thickness did not change. Meadors et al. [4] investigated the effects of thermocycle frequency, aging temperature, and post-cure conditions on weight loss, micro-hardness, and crack formation in PMR-15 neat resin. Crack formation was monitored by metallography, while molecular level changes were monitored by Fourier transform infrared spectroscopy. It was found that weight loss, crack formation, and micro-hardness were highly dependent on aging temperature and time but not on thermocycle frequency. Both the physical and the chemical effects of aging were isolated to a thin surface layer. No changes were found to occur in the interior of the samples even after 1,000 h of aging at 345 °C. Xie et al. [5] compared the thermal characteristics of PMR-15 with other novel PMR polyimides. They reported that PMR-15 showed a one-step degradation behavior, but other polyimides (TMBZ-15, DMBZ-15, and BFBZ-18) illustrated more complex decomposition behaviors. Thermogravimetric analysis in nitrogen (N2) showed that PMR-15 had the highest stability compared to the other polyimides tested. The pinpoint dynamic mechanical analysis test was used by Dole and Chauchard [6] to study the degradation of elastomeric materials. The determination of oxidation profiles at various aging times and temperatures allowed the evaluation of the apparent activation energies of the two different kinetic regimes: at the surface, the real oxidation rate, and in the bulk, kinetics limited by oxygen diffusion.
Nanoindentation is frequently used for probing polymeric [7–11] and rubbery [12] materials to investigate their mechanical properties. Over the last decade, published literature has detailed the basic principles, test procedures, and data analyses of nanoindentation testing (e.g., in [13–15]). Pharr [16] concluded that the use of nanoindentation is a resourceful technique for measuring mechanical properties of materials at and below the micron level. The elastic modulus and hardness for materials which do not pile up during nanoindentation can be measured with ±10% accuracy, but those materials that exhibit a large amount of pile up usually have overestimates of these properties by as much as 50% because of miscalculation of the indenter contact surface area.
Using an atomic force microscope, Johnson et al. [17] made nanoindentation measurements to study the effects of temperature and time on oxidation layer profiles in PMR-15 resin. Results confirmed that the surface layer and the core region had substantially different properties, and that there were two zones within the oxidized portion of the samples. Johnson et al. [17] summarized that the outer “plateau” region is the homogeneous oxidized layer, which is a result of a zero-order reaction. The transition reaction zone is the diffusion-controlled oxidation zone, which is a result of a first-order reaction. The changes in elastic moduli and oxidative layer growth have also been characterized by Ripberger et al. [18] in PMR-15 resin using nanoindentation techniques. In general, the modulus of the material in the oxidized region is higher toward its outer edge and decreases to the unoxidized modulus in the interior. The measured increase in stiffness near the specimen edge is consistent with embrittlement associated with aging the neat resin specimen in air. Closer examination of nanoindentation data reveals three distinct regions, namely the “plateau” surface layer corresponding to higher modulus values in the oxidized region, unoxidized bulk in the interior of the specimen corresponding to lower modulus values, and a transition region where the modulus reduces from the higher to the lower values. Thus, nanoindentation can be utilized for indirect measurement of the degree and the extent of oxidation.
Additionally in PMR-15, oxidation of the surface layer near the specimen edges changes the optical characteristics of the material, and using bright-field light microscopy, the oxidized material is observed to have a different appearance than the unoxidized interior. Previous work by several researchers [1–4, 18] documents the growth of the oxidized region near the exposed edges of the specimen. Figure 1 shows a photomicrograph of a PMR-15 neat resin specimen isothermally aged in ambient air at 343 °C for a period of 196 h. The figure clearly shows the oxidized region (much like a picture frame) on the two adjacent exposed free surfaces of the specimen. Between the outer oxidized layer and the interior unoxidized region is a transition region, which is the active “reaction” or “process” zone [18]. These observations allow easy measurement and characterization of the oxidized surfaces and the transition regions as a function of aging temperature and time. In our isothermal aging experiments with PMR-15 resin, we observed that an oxidized layer forms on the exposed surfaces within a short 1 h time period. The thickness of the oxidized layer increases with aging time and is seen to approach a plateau value as the oxidation growth rate reduces considerably for longer aging time periods, whereas the thickness of the active “reaction” zone remains nearly constant for the aging times considered.
Our recent work [19] shows that the specimen surface profile developed during sample preparation does not appear to affect the characterization of thermo-oxidative behavior of high-temperature PMR-15 resin using optical microscopy and nanoindentation techniques. In this study, nanoindentation and optical microscopy have been used to characterize the influence of service environment on the oxidative process in high-temperature PMR-15 resin. Neat resin specimens are isothermally aged for different time periods at several different temperatures (288, 316, and 343 °C) in a number of environments (lab air, dry air, inert gas) and under accelerated aging (elevated air pressure) conditions. The aged specimens are sectioned and prepared for optical and nanoindentation measurements using established procedures, as discussed in section “Experimental details.” Thermo-oxidative behavior is characterized by monitoring the growth of the oxidative layer and the modulus evolution within the oxidized zone and the sample interior as a function of the aging environment and time. These measurements will be subsequently incorporated into mechanistic models [20, 21] for predicting the performance of high-temperature composites under service environment.
Experimental details
Sample preparation
Rectangular PMR-15 neat resin plaques measuring 11.68 × 15.24 cm2 with an average thickness of 0.23 cm were fabricated in an autoclave using a steel die mold with Teflon coating and the material manufacturer’s suggested cure cycle. Subsequent to the plaques being post-cured in air for 16 h at 343 °C, they were cut using a diamond wet-saw with distilled water as a cooling media. All samples to be aged were then washed using a common household soap and then rinsed with distilled water for a minimum of 5 min. Rubber gloves were worn throughout while handling the sample (before and after the aging process) in order to prevent contamination of specimens from oils, etc. The specimens were then dried with standard paper towels and placed in a vacuum oven at 105 °C for a minimum of 48 h to remove any moisture within the samples, and subsequently stored in a nitrogen-purged desiccator until testing.
Aging configurations
In this research, unaged specimens and specimens aged isothermally in lab air at 288, 316, and 343 °C were studied. A typical use temperature of PMR-15 neat resin is 288 °C, however 316 and 343 °C were also chosen to evaluate the material response at temperatures above typical use temperatures. Since the use temperature of HTPMCs is often near the glass transition temperature of the material, the ability to accelerate aging by increasing the temperature is limited. The use of pressure to accelerate the oxidative aging process has been used in the aircraft engine community based in part on the fact that engine parts experience elevated pressures during use. Therefore, PMR-15 neat resin specimens were also subjected to aging in air at a pressure of 0.414 MPa (60 psi) at 288 °C in order to evaluate the effect of elevated pressure on the rate of thermo-oxidative degradation.
Additionally, specimens aged in an inert argon environment are included to show the effects of thermal aging in a nonoxidizing environment. It was previously determined from aging specimens in nitrogen that nitrogen reacts with PMR-15 at the aging temperatures of interest. Therefore, argon gas is currently used for inert aging. Lastly, the effect of the moisture in the ambient laboratory air on the aging process was determined by comparing the behavior of the specimens aged in dry air (desiccated and filtered) to the specimens aged in the ambient air. Since the ambient air aging is much less labor intensive, and thus less expensive to conduct than the dry air aging, the ambient air aging is preferable if the difference in the two aging conditions can be shown to be negligible.
Samples aged in lab air were placed on unsized quartz cloth in order to prevent the material from coming in contact with any metal parts in the oven. Prior to being used all quartz cloth was heated to 500 °C for 1 h to remove organics left over from processing. The air supply used for the oxidizing environment was ambient air fed by convection through the oven inlet. Specimens aged at 0.414 MPa in dry air were placed in an unsized quartz cloth-lined steel pressure chamber. Steel tubing attached to regulated house air (with two in-line desiccators) delivered a continuous supply of dry air, which bled the pressure chamber of ambient lab air and maintained a constant pressure of 0.414 MPa. The second desiccator contained a purifier that removed any contaminants and impurities with an effective molecular diameter of <5 Angstroms from the dry air before it entered the pressure chamber. The oven was then brought up to the specified aging temperature and the samples were allowed to age for the specified time.
At a specified time interval, a smaller specimen was dry-sectioned from the aged large sample, as seen in Fig. 2, to examine the development of the oxidized layer. This allowed monitoring of four exposed edges of the large specimen. The diamond blade used to section these samples was washed with acetone and wiped clean with paper towels to minimize the amount of contamination from the cutting wheel. After dry-sectioning smaller specimens from the large specimens, the large specimens were vacuum-dried for 24 h before being placed back into the oven to continue the aging process.
Optical microscopy/nanoindentation characterization
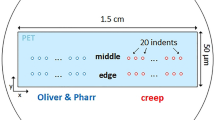
Unaged and aged PMR-15 samples, which were removed from the oven at various aging intervals, were potted in 828-D230 epoxy resin and cured at room temperature for 3 days. The specimens were then wet-sanded with 600-grit sandpaper and distilled water and polished using a 0.3-μm alumina polishing media. These potted specimens were used for both nanoindentation testing and optical microscopy measurements.
An optical microscope (Nikon Microphot-FXL, Model F84006) in the bright-field mode was used to measure the thickness of the oxidized surface layer. The intensity of the microscope light was adjusted such that the entire aged sample was well illuminated. Care was exercised to ensure that the potted specimen was flat once the oxidation layer was in view under the microscope. This was done by verifying that the layer is approximately the same thickness on all sides of the specimen. If it was not, the sample likely was cut obliquely or not mounted flat and was remade. Once it was verified that the specimen was properly mounted, the thicknesses of the oxidative layer and transition region were measured. A total of 12 measurements were made, three thickness values along each edge of the specimen and the averages were computed. These measurements were recorded and compared for the different aging conditions.
Nanoindentation tests were performed using a Nano Indenter® XP (MTS NanoInstruments, Oak Ridge, TN, USA). A high-resolution actuator was used to drive the indenter head into the test sample, while a high-resolution sensor measured the applied force and penetration depth. Since both plastic and elastic deformations occur as the indenter is pushed into the surface of the material, the elastic modulus is calculated using the slope of the unloading curve as the indenter head is withdrawn from the material surface. This portion of the unloading curve contains only the recoverable elastic portion of the displacement since plastic deformation is not recoverable. The elastic modulus of the test material, E, is calculated using the equation:
where n is the Poisson’s ratio of the test material, and ni (0.07) and Ei (1,141 GPa) are the Poisson’s ratio and elastic modulus of the indenter head, respectively. Er is the reduced modulus described by the equation:
where S is the slope of the initial portion of the unloading curve (also known as the elastic stiffness of the contact), A the projected contact area at load P calculated by an empirically determined area function at contact depth, hc, and b is a constant dependent on the indenter head geometry. The area function relates the projected area sustaining the load during indentation to the contact depth, hc. The Berkovich indenter used in this work is shaped as a three-sided pyramid at q = 65.3°, and this geometry has a b value of 1.034. The indenter tip was calibrated by the method described by Oliver and Pharr [22] using a fused silica standard, the modulus of which is 72 GPa. Pile-up (or sink-in) leads to contact areas that are greater than (or less than) the cross-sectional area of the indenter at a given depth. In such cases, measurements of contact compliance as well as direct SEM measurements of the areas of large indentations can be utilized for indenter tip-shape calibration [23]. A microscope to indenter calibration was also performed using a single-crystal aluminum sample to establish relative positioning between the microscope and indenter. When properly calibrated, indentation is performed exactly at the specified position on the sample. Indentations were made across the total oxidation thickness of each specimen for three randomly chosen arrays/paths perpendicular to each of the specimen edges. The elastic modulus was obtained based on the continuous stiffness method, which is known to improve the accuracy of the indentation test due to its dynamic nature. This technique allows for the continuous measurement of the contact stiffness during loading, not just during initial unload. This is accomplished by superimposing a small oscillation on the primary loading signal and analyzing the resulting response of the system by means of a frequency-specific amplifier. With a continuous measure of the slope of the unloading curve S, one obtains the elastic modulus as a continuous function of depth from a single indentation experiment. A review of the nanoindentation continuous stiffness measurement technique and its applications is given by Li and Bhushan [24]. Manufacturer’s suggested default values were used for system parameters such as the allowable drift rate (.05 nm/s), frequency (45 Hz), strain rate target (.05/s), harmonic displacement target (2 nm), and the surface approach distance (1,000 nm).
Results and discussion
Unaged specimen
In this study, the contact stiffness was determined by operating the nanoindenter in the dynamic mode. Thus, one obtains elastic modulus as a continuous function of depth from a single indentation experiment. Figure 3 shows an example of the elastic modulus as a function of depth for the baseline unaged PMR-15 resin. The larger stiffness values calculated near the specimen surface corresponding to the initial stages of penetration are probably an artifact of the imperfect surface contact at small depth values [25]. However, the contact stiffness becomes relatively independent of position beyond a depth >200 nm and converges to a plateau value. It is this constant value of contact stiffness that is reported for the work conducted here. An average elastic modulus of 4.84 GPa (with a standard deviation of 0.24 GPa) was obtained using data from three different scans in various regions of the resin specimen (for a total of 80 indents) for unaged PMR-15 resin, as shown in Fig. 4.
Mechanical testing of unaged PMR-15 coupon specimens reveals that the resin is stiffer in compression, and that the compressive modulus of unaged PMR-15 resin decreases from 4.72 to 3.39 GPa due to temperature rise from 25 to 288 °C. Thus, there is a reasonably good agreement between the values obtained from the coupon testing (4.72 GPa at room temperature) and those obtained from the nanoindentation (4.84 GPa) for the unoxidized resin.
Aging in an inert environment
As mentioned earlier, PMR-15 resin specimens were aged in an inert argon environment to examine the effects of thermal aging in a nonoxidizing environment. Figure 5 compares the average elastic modulus obtained on specimens aged in argon for various time periods at 288 °C with the unaged resin specimens. Each one of the datum points is an average of at least 40 individual indents, while the standard deviations on the measurement are shown as error bars. The elastic modulus of the resin aged in argon is observed to increase slightly over the unaged value, although the increase seems to be relatively independent of the aging time.
In Fig. 5, we have also plotted the average modulus measured in the interior (i.e., in the unoxidized region) of the specimens aged in lab air at 288 °C. Because oxidation is diffusion driven, the interior of the specimens aged in an oxidative environment is not exposed to oxygen and therefore has properties similar to that of the specimens aged in the oxygen-depleted (or inert) environment. It is seen that the average modulus in the unoxidized region of the air-aged sample is approximately equal to that of the average modulus of the sample aged in the unoxidizing argon atmosphere. These results therefore are consistent with the assumptions of Tsuji et al. [2] that the properties of the specimens aged in an unoxidizing environment can be taken to be similar to the properties of the unoxidized core material in the air-aged specimens.
Aging in an oxidizing environment
PMR-15 samples aged in air were scan-indented across the oxidized layer, through the active “process” or “reaction” zone (defined as the transition region between the oxidized and the unoxidized areas of the aged specimens), and into the unoxidized interior of the specimen. The indents were spaced so as to give a minimum of three data points within each region. These measurements were repeated at several locations around the perimeter of the oxidized samples. Scatter exists in the data as a result of sampling through different regions of the specimens and also from individual scans. This is indicative of the spatial variability and the heterogeneous nature of the degradation process. In general, the modulus of the material in the oxidized region is higher toward its outer edge and decreases as the indenter head propagates into the interior. The measured increase in stiffness near the specimen edges is consistent with embrittlement associated with aging the neat resin specimens in air.
Influence of aging temperature
Figure 6 compares the elastic modulus profiles of samples isothermally aged at 288, 316, and 343 °C for ~50 h. The graphs in Fig. 6a–c show the variation of elastic moduli from three individual scans for each temperature as a function of distance from the specimen edge, where the oxidative layer begins. The horizontal dashed line in these figures is the average unaged value. Note that each individual scan results in two data sets—one measured from the left (or top) edge of the sample and the other is from the right (or bottom) edge. Close examination of nanoindentation data reveals three distinct regions: the surface layer corresponding to higher modulus values in the oxidized region, unoxidized bulk in the interior region of the specimen corresponding to lower unaged modulus values, and a transition region where the modulus reduces from the higher stiffness surface values to the lower stiffness interior values. The increased stiffness in the oxidized region near the specimen surface is a manifestation of the chemical changes in the polymer occurring during oxidation. Using optical micrographs, measurements were also made of the oxidized layer and transition region thickness at several locations. The average values of these measurements are plotted as dotted vertical lines in Fig. 6a–c. It is observed that the average thickness of the oxidation layer and active “reactive” region measured by optical methods are in good agreement with the boundaries of three regions suggested by the nanoindentation data.
It is seen that the average elastic modulus in the oxidized region and the thickness of the oxidized region remain relatively insensitive to variations in aging temperature. However, the thickness of the transition region is found to decrease with increase in aging temperature since the oxidation reaction rate increases with increase in temperature resulting in a smaller zone over which active reactions take place.
Influence of aging time
The development and growth of the oxidative layer was also measured as a function of the aging time. Figure 7 shows the results of the modulus measurements made on PMR-15 resin aged at 288 °C for (a) 48 h, (b) 541 h, and (c) 1,556 h of isothermal exposure. Similar to Fig. 6, the average values of optical measurements of the oxidized layer and transition zone thickness are plotted as dotted vertical lines in Fig. 7, and are in good agreement with the boundaries of the regions suggested by the nanoindentation data. As expected, there is an increase in the thickness of the oxidation layer with aging time. However, the rate of growth decreases and eventually approaches an auto-retardation state. It is likely that oxidation continues to occur in the oxidized region but at a much slower rate than in the transition region. On the other hand, the thickness of the transition zone remains approximately constant with aging time. The average elastic modulus in the oxidized region is also relatively insensitive to variations in aging time. Thus, as the material oxidizes it converts to a brittle material, and minimal change in material modulus occurs with further aging time.
Influence of humid environment
The effects of moisture in the ambient laboratory air on the aging process was determined by comparing the behavior of the specimens aged in dry air (desiccated and filtered) to the specimens aged in the ambient air. Figure 8 compares the results of the modulus measurements made on PMR-15 resin aged at 288 °C for 541 h at ambient pressure in (a) lab air and (b) in dry air. There is no significant difference in thickness of the oxidized region and the transition zone for specimens aged at ambient pressure in dry air and ambient air. Moreover, the average elastic modulus in the oxidized region is relatively insensitive to the variation in aging environments considered. Although it is well known that PMR-15 is highly susceptible to hydrolytic degradation [26], the ambient lab air did not appear to contain enough moisture to affect the results of the present aging study. Since the difference in the two aging conditions is shown to be negligible for the measurement techniques used, the ambient air aging was used in subsequent testing.
Influence of aging pressure
The use of pressure to accelerate the oxidative aging process was examined by comparing the behavior of specimens aged at an elevated pressure to specimens aged at ambient pressure in lab air. Figure 9 compares the results of the modulus measurements made on PMR-15 resin aged at 288 °C for (a) 541 h at ambient pressure, and (b) 571 h at 0.414 MPa (60 psi). The elevated pressure aging clearly increases the thickness of the oxidative layer as compared to ambient pressure-aged specimens. The pressurization of the air during aging increases the rate of diffusion of the oxygen into the neat resin resulting in a substantial increase in oxidation layer growth rate. However, the thickness of the transition zone remains relatively insensitive to the variation in aging pressure. Moreover, there is little difference in the modulus of the aged resin with aging pressure. Thus, there is an accelerated growth of oxidation on aging under elevated pressure at the use temperature. It is anticipated that by aging the specimens below their glass transition temperature, the reaction rates and chemical mechanisms remain approximately the same as those likely to be experienced by a part in actual use, while scaling of the lifetime of such parts may be achieved by accelerating oxidation using elevated pressure [26].
Conclusions
In this work, nanoindentation and optical microscopy techniques have been used to characterize the thermo-oxidative behavior of high-temperature PMR-15 resin aged isothermally under a wide range of service environment conditions. Specifically, the influence of aging temperature, time, humidity and pressure on the development, and growth of oxidation is studied. The extent of oxidation, manifested as changes in elastic moduli measured using nanoindentation, and changes in optical characteristics observed using bright-field microscopy techniques, is determined near the surface regions of aged specimens. It is shown that the average thickness of the oxidation layer and active “reactive” region measured by optical methods are in good agreement with the boundaries of three regions suggested by the nanoindentation data.
For this study, the effect of aging time, temperature, humidity and pressure on measured oxidation thickness, and elastic modulus of isothermally aged PMR-15 neat resin was investigated. Specific observations/results from the study are listed here.
-
Aging in an inert environment leads to a small increase in resin modulus over the unaged value and is similar to the modulus measurements made in the interior of the specimens aged in an oxidative environment.
-
As aging time increases, the oxidation layer thickness initially and then approaches an auto-retardation state. The thickness of the transition zone remains approximately constant, while the modulus of the oxidized material does not change significantly with aging time.
-
For the temperatures investigated, increasing temperature minimally effects the oxidation layer thickness growth rate. However, the thickness of the transition region decreases with an increase in aging temperature since the oxidation reaction rate increases with increase in temperature.
-
There is no significant difference in growth rate of oxidation for samples aged in dry air and ambient air. Even though PMR-15 is highly susceptible to hydrolytic degradation, the ambient lab air does not contain enough moisture to affect the measurable oxidation layer thickness.
-
Elevated pressure aging at the use temperature increases the thickness growth rate of the oxidation layer without affecting the size of the transition zone. This is an important consideration since elevated pressure is experienced by some HTPMC components in their service environments.
-
The average elastic modulus in the oxidized region is relatively insensitive to variations in aging temperature, time, and the environments.
It is the measurement of the aging-dependent evolution of the constituent properties and extent of oxidation and size of the transition zones that can be incorporated into mechanistic models [20, 21] for predicting the durability and lifetime performance of high-temperature resins and their composites under the service environment.
References
Bowles KJ, Papadopoulos DS, Inghram LL, McCorkle LS, Klan OV (2001) NASA/TM-2001-210602
Tsuji LC, McManus HL, Bowles KJ (1998) NASA Technical Report -208487:1
Régnier N, Berriot J, Lafontaine E, Mortaigne B (2001) Polym Degrad Stab 73:485
Meadors MA, Lowell CE, Cavano PJ, Herrera-Fierro P (1996) High Perform Polym 8:363
Xie W, Pan WP, Chuang KC (2001) Thermochim Acta 367–368:143
Dole P, Chauchard J (1995) Polym Degrad Stab 47:441
Briscoe BJ, Fiori L, Pelillo E (1998) J Phys D Appl Phys 31:2395
Raghavan D, Gu X, Nguyen T, VanLandingham M, Karim A (2000) Macromolecules 33:2573
Gillen KT, Clough RL, Quintana CA (1987) Polym Degrad Stab 17:31
Gregory JR, Spearing SM (2005) Compos Sci Technol 65:595
Parvatareddy H, Wang JZ, Lesko JJ, Dillard DA, Reifsnider KL (1996) J Compos Mater 30(2):210
Gillen KT, Terrill ER, Winter RM (2001) Rubber Chem Technol 74(3):428
Oliver WC, Pharr GM (1992) J Mater Res 7(6):1564
Pharr GM, Bolshakov A (2002) J Mater Res 17(10):2660
Hay JL, Pharr GM (2000) ASM handbook. ASM International, Materials Park, p 232
Pharr GM (1998) Mater Sci Eng A253:151
Johnson LL, Eby RK, Meador MAB (2003) Polymer 44:187
Ripberger E, Tandon GP, Schoeppner GA (2004) In: Proceedings of the SAMPE 2004 symposium/exhibition, Long Beach, CA, 16–20 May
Putthanarat S, Tandon GP, Schoeppner GA (2007) Polym Degrad Stab 92:2110
Tandon GP, Pochiraju KV, Schoeppner GA (2006) Polym Degrad Stab 91(8):1861
Schoeppner GA, Tandon GP, Pochiraju KV (2007) In: Kwon Y, Allen D, Talreja, R (eds) Multiscale modeling and simulation of composite materials and structures. Springer, New York
Oliver WC, Pharr GM (2004) J Mater Res 19(1):3
McElhaney KW, Vlassak JJ, Nix WD (1998) J Mater Res 13(5):1300
Li X, Bhushan B (2002) Mater Charact 48:11
Mencik M, Swain MV (1995) J Mater Res 10(6):1491
Thorp KE (2000) PhD Dissertation, University of Dayton, Dayton
Acknowledgements
This work is supported by the Air Force Office of Scientific Research under the Materials Engineering for Affordable New Systems (MEANS-II) program sponsored by Dr. Charles Lee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Putthanarat, S., Tandon, G.P. & Schoeppner, G.A. Influence of aging temperature, time, and environment on thermo-oxidative behavior of PMR-15: nanomechanical characterization. J Mater Sci 43, 6714–6723 (2008). https://doi.org/10.1007/s10853-008-2800-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2800-1