Abstract
Acidic hydrothermal degradation of glucose was examined in the presence of HCl, H2SO4, and H3PO4 with pH varying from 1.5 to 2.5 and reaction time varying from 1 to 10 min at 523 K, to investigate the effect of different acid catalysts and acid concentration on the production of 5-hydroxymethyl-2-furaldehyde (HMF) and levulinic acid from glucose. At lower acidities of pH 2.5, a considerably higher amount of HMF was produced. The increase in acid concentration accelerated the conversion of HMF to levulinic acid. The order for the production of HMF using the three acids is in the sequence of H3PO4 > H2SO4 > HCl. On the contrary, the order for production of levulinic acid follows HCl > H2SO4 > H3PO4. In the experimental conditions used in this study, the highest yield of levulinic acid is about 55%, which was obtained at pH 1.5 for 5 min in the case of HCl as an acid catalyst, and the total highest yields of HMF and levulinic acid are about 50%, which occurred at pH 2.0 for 5 min in the case of H3PO4 as an acid catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The conversion of biomass into resources has gained considerable attention for maintaining the global carbon cycle system to work harmoniously. Hydrothermal treatment is one of the most effective methods among several processes for conversion of biomass into resources, because water under high temperature and high pressure behaves as a reaction medium having outstanding properties.
5-Hydroxymethyl-2-furaldehyde (HMF) and levulinic acid are important chemicals. In the carbohydrate chemistry, it is well known that HMF is a product of the facile, acid-catalyzed dehydration of carbohydrate, and levulinic acid is a product from a subsequent rehydration reaction of HMF. Previous researches [1, 2], including our studies [3, 4], have found that HMF was a significant product under hydrothermal treatment of carbohydrates probably because water in the subcritical region acts as an effective acid catalyst. Furthermore, many researchers also reported that acid catalysts could accelerate the production of HMF and levulinic acid under hydrothermal condition [5, 6]. Most of these researchers mainly focused on the production of only HMF or only levulinic acid. Few of them reported the effect of acid catalysts on the total production of HMF and levulinic acid. Also, few studies, except for a recent study reported by Girisuta et al. at [6], examined the effect of different acid catalyst on the production of HMF and levulinic acid.
Our previous research on wet oxidation (WO) of carbohydrate biomass to acetic acid showed that the yield of acetic acid was increased greatly by a two-step process, consisting of both a hydrothermal reaction without a supply of oxygen (the first reaction step) and an oxidation reaction (the second reaction step). The first step is to accelerate the formation of HMF and lactic acid since they can produce a large amount of acetic acid by their oxidation, and the second step is to further convert these products formed in the first step to acetic acid by the oxidation with newly supplied oxygen. Our recent studies have shown that the acetic acid yield from the oxidation of levulinic acid was higher than that from HMF. So, it is expected that the acetic acid yield could be further enhanced by adding acid catalysis for improving the production of levulinic acid in the first reaction step.
In this paper, therefore, we investigated the effect of different acid catalysts of HCl, H2SO4, and H3PO4 not only on the production of HMF and levulinic acid, but also on the total yields of HMF and levulinic acid, by varying the concentration of the acid catalysts.
Experimental
Materials
Glucose, a model compound of carbohydrate biomass, was chosen as test material. Reagent grade 5-hydroxymethyl-2-furaldehyde (HMF) and levulinic acid were also used as test materials. HCl, H2SO4, and H3PO4 were used as acid catalysts. Amount of starting materials was limited to 0.07 g (dry base).
Experimental procedure
All experiments were carried out using a batch reactor with an internal volume of 5.7 mL, constructed of a piece of stainless steel 316 tube (3/8 inch diameter, 1 mm wall thickness, and 120 mm length). To keep the reaction in a single phase, water fill was fixed at 50%, which was defined as the ratio of the volume of water added into the reactor and the inner volume of the reactor. The typical reaction procedure was as follows: The test material and water or aqueous solutions of acid catalyst were added into the reactor and sealed thereafter. The reactor was immersed into a preheated salt bath for the reaction to occur. The reactor was vibrated and agitated during the reaction. After the reaction time elapsed, the reactor was removed from the salt bath and put into a cooling bath to quench the reaction. The reaction time was defined as the duration for which the reactor was kept in the salt bath. All experiments were performed at a temperature of 523 K. Reaction time varied from 1 to 10 min. The initial pH was varied to 2.5, 2.0, 1.5.
Analytical methods
After cooling, the liquid sample was filtered through a 0.45 μm filter, and then diluted 10 times with distilled water. Liquid samples were analyzed by gas chromatography flame ionization detector (GC-FID) and high-performance liquid chromatography (HPLC). For GC-FID analyses, a Hewlett-Packard model 5890 Series II Gas Chromatograph equipped with a flame ionization detector with a HP-INNOWAX capillary column (Cross-Linked Polyethylene Glycol) was used for water-soluble compounds, using helium as the carrier. HPLC analysis was performed with a Waters HPLC system equipped with a tuneable absorbance detector (UV/VIS detector) (Waters 486).
Results and discussion
Effect of different acid catalysts on the production of HMF and levulinic acid at lower acidities of pH 2.5
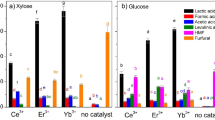
Figure 1 shows the yields of HMF and levulinic acid from glucose by acid catalytic hydrothermal reaction for pH 2.5. Results obtained in the absence of acid catalysts are also shown in Fig. 1 for comparison. Yield of the product is defined as follows.
It can be seen that, from Fig. 1, the three acid catalysts improved the production of HMF compared to the results in the absence of acid catalyst. The yield of HMF increased with increasing reaction time, and decreased beyond 5 min for the three acid catalysts. In the case of HCl, the decrease in the yield of HMF was significant, compared to H3PO4. The catalytic effect of H2SO4 and H3PO4 was higher than that of HCl in enhancing the formation of HMF, with a considerable yield of 40% at 5 min.
On the other hand, the yield of levulinic acid was quite low for all the three acid catalysts. The lower yield of levulinic acid may be due to the conversion of HMF to 1,2,4-benzentriol [7]. Intermediate products were identified by HPLC, to examine the production of 1,2,4-benzentriol. As shown in Fig. 2, 1,2,4-benzentriol was found. Although the peak of 1,2,4-benzentriol is considerably high, quantitative analysis showed that the yield of 1,2,4-benzentriol was only 2–4% (see Table 1). These results show that glucose is easily converted to HMF with a higher yield even at lower acidities. However, HMF is not easily converted into levulinic acid at lower acidities. In other words, more-acidic conditions are needed for the conversion of HMF to levulinic acid.
Effect of different acid catalysts on the production of HMF and levulinic acid at higher acidities
Figure 3 shows the yields of HMF and levulinic acid by acid catalytic hydrothermal reaction of glucose in the case of pH 2.0. Compared to pH 2.5, the yield of HMF at a shorter reaction time of 1 min was higher for all the acid catalysts, particularly in the case of HCl. After 1 min, the variation of HMF with reaction time was similar to that at pH 2.5 for both H2SO4 and H3PO4. The yield of HMF initially increased then decreased as reaction time increased, and the highest yield of HMF was obtained at 5 min. However, in the case of HCl, the HMF yield only decreased with the increase in reaction time. These results suggest that the increase in the concentration of acids improved not only the formation of HMF but also the decomposition of HMF. Decomposing effectiveness of HCl on HMF was strongest among the three acid catalysts.
The formation of levulinic acid was observed obviously for the three acids at pH 2.0, indicating that the produced HMF was converted to levulinic acid. At the same time, these results also indicated that higher acidities are needed for the rehydration reaction of HMF to levulinic acid. For HCl, the yield of levulinic acid evidently increased with the increase in reaction time. As mentioned before, the yield of HMF decreased greatly with the increase in reaction time in the case of HCl. This suggests that catalytic effect of HCl for the conversion of HMF to levulinic acid was higher than that of H2SO4 and H3PO4.
Although the respective yields of HMF and levulinic acid were not very high at pH 2.0 for all the three acid catalysts, for the case of H3PO4 at 5 min, the total yields of levulinic acid and HMF were considerably high, which reached 50%. As mentioned before, a two-step process can greatly increase the acetic acid yield. The first step is to accelerate the formation of HMF and lactic acid without the addition of any catalysis since HMF and lactic acid can produce a large amount of acetic acid by their oxidation. The second step is to further oxidize HMF and lactic acid to acetic acid. Our recent results also showed that the acetic acid yield by the oxidation of levulinic acid is higher than that by the oxidation of HMF. So, it is expected that the acetic acid yield could be further enhanced by adding acid catalysis for improving the production of levulinic acid in the first reaction step. In the case of production of acetic acid by acid two-step process (with the addition of catalyst in the step reaction), the use of pH 2.0 may be better than that of pH 2.5.
Further, experiments with a higher concentration of acids of pH 1.5 were performed. As shown in Fig. 4, the yield of HMF greatly decreased and become almost zero at 10 min for all the acid catalysts. On the contrary, further increase in the concentration of acids leads to further an increase in levulinic acid. These observations show that the increase in acid concentration improve the conversion of HMF to levulinic acid. Similar to the results at pH 2.0, the yield of levulinic acid increased with the increase in reaction time. For HCl, the yield of levulinic acid was highest and reached about 55%.
From the above results, it can be clearly seen that, the order for the production of HMF using three acids is in the sequence of H3PO4 > H2SO4 > HCl. On the contrary, the order for production of levulinic acid follows HCl > H2SO4 > H3PO4. Both the dehydration of glucose into HMF and the rehydration of HMF into levulinic acid are acid-catalyzed reactions. In general, if the acid is stronger, the acid-catalyzed reaction is easer to take place. The observed order for the production of levulinic acid is the same as this expectation. However, for the production of HMF from glucose, the reversed order was observed. A possible explanation would be as follows.
The generally accepted mechanism for acid-catalyzed dehydration of alcohols is the following:

In the dehydration, acid is needed to convert the alcohol into the protonated alcohol, which can then undergo heterolysis to lose the weakly basic water molecular. Acid transforms the very poor leaving group, –OH, into the very good leaving group, –OH +2 , and acid is not consumed. So, for the highly reacting alcohols, the dehydration occurs easily by the presence of only trace amounts of acid. Since glucose is a highly reacting polyalcohol, by using a weaker acid such as H3PO4, the formation of a considerably higher amount of HMF is possible. However, when using a strong acid such as HCl, as mentioned before, it can accelerate the rehydration of HMF into levulinic acid. Thus, the observed order for the production of HMF from glucose was in the sequence of H3PO4 > H2SO4 > HCl. For the rehydration of HMF into levulinic acid, since levulinic acid is very stable, the order for the production of levulinic acid agree with the expectation that the stronger the acid, the higher is the yield of levulinic acid.
Conclusions
At lower acidities of pH 2.5, a considerably high amount of HMF was produced. The increase in acid concentration accelerated the conversion of HMF to levulinic acid. The catalytic effect of H2SO4 and H3PO4 was higher than that of HCl in the conversion of glucose to HMF, whereas HCl is more effect than H2SO4 and H3PO4 for further conversion of HMF into levulinic acid.
Under the conditions used in this study, the highest yield of levulinic acid is about 55%, which occurred at pH 1.5 for 5 min in the case of HCl as an acid catalyst. The total highest yield of HMF and levulinic acid are about 50%, which occurred at pH 2.0 for 5 min in the case of H3PO4 as an acid catalyst.
References
Kallury RKMR, Ambidge CA, Tidwell TT, Boocock DGB, Agblevor FA, Stewart DJ (1986) Carbohydrate Res 158:253
Srokol Z, Bouche A, van Estrik A, Strik RCJ, Maschmeyer T, Peters JA (2004) Carbohydrate Res 339:1717
Jin F, Zheng J, Enomoto H, Moriya T, Sato N, Higashijima H (2004) Chem Lett 33:126
Jin F, Zhou Z, Moriya T, Kishida H, Higashijima H, Enomoto H, (2005) Environ Sci Technol 39:1893
Asghari FS, Yoshida H (2006) Industr Eng Chem Res 45:2163
Girisuta B, Janssen LPBM, Heeres HJ (2006) Chem Eng Res Design 84:339
Luijkx GCA, van Rantwijk F, van Bekkum H (1993) Carbohydrate Res 242:131
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeuchi, Y., Jin, F., Tohji, K. et al. Acid catalytic hydrothermal conversion of carbohydrate biomass into useful substances. J Mater Sci 43, 2472–2475 (2008). https://doi.org/10.1007/s10853-007-2021-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-2021-z