Abstract
High purity gallium oxide nanopowders have been synthesized by using a simple precipitation technique with calcination at elevated temperature. From the X-ray pattern, the phase purity of the synthesized powders was confirmed as β-Ga2O3. Elemental quantification (stoichiometry) of Ga2O3 was also examined from the X-ray energy dispersive analysis (EDAX). Based on the recorded Fourier Transform Infrared (FTIR) spectrum of Ga2O3, the IR bands due to Ga–O bond and crystal lattice vibrations have been identified in the wavenumber range 400–4,000 cm−1. From the measured SEM images, it is obvious to notice that the pH value has been playing a dominant role in obtaining morphologically different gallium oxide nanopowders. Thermogravimetric analysis reveals 8.3% of weight loss when the sample was heated to the temperature of 1,100 °C from the room temperature, which also shows a crystalline phase transformation. It is very interesting to report that a broad blue emission at 455 nm has been measured from the synthesized gallium oxide nanopowders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal oxides exist in different compounds and crystalline structures, and their properties could vary widely from insulators to superconductors resulting in a wide variety of potential applications. Monoclinic gallium oxide (β-Ga2O3) has got a wide band gap (4.9 eV) amongst the semiconductor metal oxides. Gallium oxide has recently been considered as a precursor in the preparation of phosphor materials in order to produce desired colors from the flat panel displays (FPDs). Gallium oxide has been found to be a material for the development of mixed Ga2O3–Al2O3 catalyst, Ga2O3 based glasses are among the best optical materials based on the advanced glass technologies. Like SnO2, ZnO gas sensors, Ga2O3 will also respond well for both oxidizing and reducing gases. At elevated temperatures (T > 900 °C) Ga2O3 thin films will show significant conductance response to oxygen gas and at lower temperatures the sensitivity switches to reducing gases such as CO, CH4 and NH3. Gallium (III)-oxyhydroxide or amorphous gallium (III)-(hydrous) oxides are suitable precursors in the synthesis of crystalline (III)-oxide. The chemical, a microstructure and physical property of Ga2O3 depends on the properties of its precursor, and therefore it is possible to control the properties of Ga2O3 by varying the precipitation chemistry of the concerned precursor. β-Ga2O3 is one of the five well known forms of gallium oxides α, χ, δ, Є, β-Ga2O3 and their polymorphs are converted to β-Ga2O3 at temperature, T > 850 °C [1]. Yeh’s group did report on the preparation of the spindle like GaOOH nanoparticles in cetyltrimethyl ammonium bromide water solution by means of a laser ablation. Zhang et al. did synthesize nanorods of β-Ga2O3 by hydrothermal synthesis of GaOOH followed by a calcination procedure at elevated temperatures [2]. Cheng and Samulski reported on the Ga2O3 nanotubes by immersing alumina membrane in amorphous Ga2O3.nH2O solution followed by drying and then heating it at 500 °C [3]. Gallium Oxide nanowires were obtained by evaporation of gallium nitride in the argon/oxygen flow [4], arc-discharge method or laser ablation [5]. Nano sheets and nanobelts of Ga2O3 were prepared via evaporation of GaN powders at 1,100 °C in an oxygen ambient atmosphere [6]. Xiang et al. reported on the preparation of β-Ga2O3 long 1-D nanowires and 2-D nanosheets by means of thermal evaporation of Ga/Ga2O3 powders in the presence of Au catalyst [7]. The first experimental study on the transformation of gallium oxide polymorphs and hydroxides was carried out by Laubengayer et al. over temperature range of 110–1,000 °C [8]. In the present paper we report on the synthesis and characterization of β-Ga2O3 nanopowders and studied the effects of pH value on the particle sizes.
Experimental
A simple and economically viable and more convenient method was adopted in the synthesis of β-Ga2O3 by precipitation followed by calcination at elevated temperatures. 5N pure gallium metal, sub-boiled nitric acid and ammonia were used as the starting chemicals. Initially, gallium was dissolved in sub-boiled nitric acid in a teflon beaker by warming it slightly. After a complete dissolution of gallium metal, an equal volume of aqueous diethylene glycole (DEG) was added to it by drop wise with a vigorous stirring. The resultant solution was divided into three equal parts, and each part was reacted with ammonium hydroxide by means of a continuous stirring. The pH value of three batches were maintained as 6, 7 and 8, separately. A dense gallium hydroxide precipitation was formed and it was later transferred into a teflon lined SS autoclave and treated it hydrothermally at 180 °C for 24 h. Hydrothermal treated mass was washed thoroughly with deionized water and it was dried in an oven. The dried gallium hydroxide mass was transferred into a quartz boat and it was subjected to a thermal treatment from room temperature to 850 °C in an electrical tubular furnace and it was kept at 850 °C for 2 h in an ambient atmosphere. The phase purity of the synthesized mass was analyzed on a Phillips X-ray diffractometer (Fig. 1), in the diffraction range of 2θ = 10–70°. The elemental quantification and stoichiometry of gallium oxide were conformed by X-ray Energy Dispersive Analysis (SEM-EDAX) (Fig. 2). The Fourier Transform Infrared (FT-IR) transmission spectrum of synthesized gallium oxide was recorded on a Nicolet IR-200 spectrophotometer using KBr pellet from 4,000 cm−1 to 400 cm−1 (Fig. 3). Thermogravimetric investigation of Ga2O3 powder was carried out from a Metler Toledo TGA/SDTA 851e (Fig. 4). 3.1175 mg of Ga2O3 sample was heated from room temperature to 1,100 °C at a heating rate of 10 °C/min. in a dynamic inert argon atmosphere with 50 ml/min. gas flow rate. The surface morphological features (shape and grain size) of produced gallium oxide with different pH values were studied by Philips XL 30, Scanning Electron Microscope (SEM). For the SEM analysis (Fig. 5a–e), Ga2O3 powders were pre-coated with a thin layer of gold in a Polaran SC 7610 sputter coater in order to prevent the charging of the specimen. The instrumental parameters, accelerating voltage, spot size, magnification and working distance are indicated on the SEM images. Photoluminescence excitation and emission spectra were measured on a Hitachi Spectrofluorimenter attached with a 150 W Xe-arc lamp as the excitation source (Fig. 6).
Results and discussion
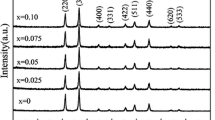
X-ray diffraction pattern (Fig. 1) of calcined gallium oxyhydroxide at 850 °C demonstrates a stable phase of β-Ga2O3 with cell parameters a = 12.23, b = 3.04, c = 5.80 Å, β = 103.7° and space group of C2/m. Irrespective of the synthesis routes both precipitated and hydrothermal synthesis followed by calcination at 850 °C have resulted in with β-phased gallium oxide powders. Quantitative analysis of produced gallium oxide was done by EDAX (Fig. 2), where it indicates Ga2O3 phase approximately with a small quantity of carbon impurity. Carbon impurity exists because of the usage of acetone as the solvent in spreading the powder uniformly on sample holder in order to obtain better SEM pictures without any agglomeration. FT-IR, transmittance spectrum was recorded in the range of 4,000–400 cm−1 (Fig. 3). The band at 3430 cm−1 reveals H–O–H stretching band and a band at 1,610 cm−1 indicates –OH bending. A strong band at 2,335 cm−1 and a weak band at 1,384 cm−1 could be due to an adsorbed atmospheric CO2, which results from the preparation and processing of FTIR samples in the ambient atmosphere. The strong bands originated at 668.3 and 455.6 cm−1 are due to Ga–O vibrations. Ristic et al. have studied the FT-IR spectrum of nanoparticle β-Ga2O3 in the wavenumber range 200–4,000 cm−1 where they have reported that IR bands could be due to Ga–O bond and crystal lattice vibrations [9]. The thermogram of gallium oxide (Fig. 4), was measured from room temperature to 1,100 °C in an dynamic inert and it is already calcined at 850 °C for two hours at the ambient atmosphere of Ar. From TG/DTA analysis the weak endothermic peak at around 410 °C indicates the elimination of adsorbed moisture while exposed to ambient atmosphere. The broad exothermic peak at 840 °C may be due to the crystallization phase transformation changes taking place in the sample. It was observed 8.3% weight loss after heat treatment compared to the initial weight [10]. From the literature it is obvious that only a very few researchers have been studied the TG analysis of gallium oxide, where it depends mostly on the method of preparation and the starting ingredients of chemical reaction. The phase transformation changes of GaO(OH) to β-Ga2O3 was observed by Laubengayer et al. and other researchers [11 – 17].
Figures 5a–e display the SEM images of gallium oxide powders synthesized at various pH values. Here the mol. concentration of gallium nitride, hydrothermal synthesis time and temperature were maintained constant. In the range of pH = 6–8, a GaOOH precipitate appeared and the system appearing to be turbid. The existence of DEG probably hinders the growth of GaOOH crystals along the direction perpendicular to the C-axis at specific pH value. Here gallium oxide powders were synthesized with three pH values i.e 6, 7 and 8 with pH 6.0 gallium oxide powders are found to be in quadrilateral spindle like structure (Fig. 5a) with an average particle width as 600 nm and length as 2.64 microns. With pH value 7.0, the gallium oxide powders (Fig. 5b) are in irregular morphology with an average particle size of 1.6 microns. Gallium oxide powders synthesized with pH value 8.0 were calcined at 600 °C (Fig. 5c) and 850 °C (Fig. 5d) where they are noticed to be in agglomerations of the nanoparticles with an average particle size of 22 nm as shown in the Fig. 5e. Comparison of the gallium oxide powders that were synthesized at 600 °C and 850 °C upon maintaining the pH and calcination duration, it has been interesting to notice that the agglomerated particles obtained at the elevated temperatures got slightly separated out. From the SEM images it is clearly observed that the powders synthesized at pH 6.0 have obtained regular shaped spindle like structures of gallium oxide. The pH value change in water has resulted in different nucleation rates, which has in turn further produced different structures in the produced gallium oxide powders; at higher pH values (pH 7–8) the nucleating process could take place faster and more crystalline nuclei could also be forming.
Under a short UV excitation, the as—GaOOH powders did not show any luminescence, but the calcined samples (β-Ga2O3) did display a strong blue emission. Figure 6, Shows the photoluminescence spectrum of the β-Ga2O3 with an excitation at 265 nm at the room temperature, where a broad blue emission band ranging from 400 nm to 550 nm with a maximum at 455 nm is observed. This is similar to that of β-Ga2O3 nanobelts [18]. The luminescence mechanism in β-Ga2O3 could be attributed to the recombination of an electron on a donor formed by oxygen vacancies with a hole on an acceptor formed by either gallium vacancies or gallium oxygen vacancy pairs [19]. Upon excitation with 265 nm, an electron could be excited from the valence band (VB) to the conduction band (CB). The electron moves freely in the CB and finally relaxes to the donor band (oxygen vacancies). The recombination of the electron in the donor band with the acceptor (gallium vacancies or gallium–oxygen vacancy pairs) could yield to the display of blue emission at 455 nm. Since the β-Ga2O3 nano-particles were developed from GaOOH precipitation method at high temperature, the loss of –OH ions could be resulting in with a number of O vacancies and Ga–O vacancy pairs.
Conclusions
Different morphological high purity gallium oxide nanopowders were synthesized by employing a simple precipitation technique. The phase purities of the produced powders were confirmed based on the profiles of XRD and EDAX. From the recorded FT-IR spectrum of Ga2O3, the IR bands due to Ga–O bond and crystal lattice vibrations were identified in the wavenumber range of 400–4,000 cm−1. From the measured SEM images, it was observed that the pH value did play a dominant role in obtaining different morphological gallium oxide powders. Especially with pH value 6.0 spindle like structured gallium oxide powders were obtained. From the photoluminescence spectrum a broad blue emission at 455 nm was measured and which significantly encourages in identifying its potentialities for different applications in certain optoelectronics devices as a blue emitting material.
References
Tas AC, Majeswski PJ, Aldinger F (2002) J Am Ceram Soc 85(6):1421
Zhang J, Liu Z, Lin C, Lin J (2005) J Crystal Growth 280:99
Cheng B, Samulski ET (2001) J Mater Chem 11:2901
Zhang J, Jiang F (2003) Chem Phys 289:243
Hu JQ, Li Q, Meng XM, Lee CS, Lee ST (2002) J Phys Chem B106:9536
Dai ZR, Pan ZW, Wang ZL (2002) J Phys Chem B106:902
Xiang X, Cao CB, Zhu HS (2005) J Crystal Growth 279:122
Laubengayer AW, Engle HR (1939) J Am Chem Soc 61:1210
Ristic M, Popovic S, Music S (2005) Mater Lett 59:1227
Berret P, Berthet P (1997) J Phys III France 7:483
Sato T, Nakamura T (1982) J Chem Tech Biotechnol 32:469
Pokrovski GS, Diakonov II, Benezeth P, Gurevich VM, Gavrichev KS, Gorbunov VE, Kandurand JL, Schott J, Khodakovsky IL (1997) Eur J Mineral 9:941
Ahman J, Svensson G, Albertsson J (1996) Acta Crystalogr C52:1336
Bashmakov IA, Kalinichenko YV, Platon VV, Rakhlin MY, Rodionov VE (1992) Inorg Mater 28:419
Fleischer M, Hollbauer L, Born E, Meixner H (1997) J Am Ceram Soc 80:2121
Choi WB, Lee NS, Kim JM (2000) Adv Mater 12:746
Huang CC, Yeh CS, Ho CJ (2004) J Phys Chem B108:4940
Zhang J, Jiang FH, Zhang LD (2004) Phys Lett A322:201
Binet L, Gourier D (1998) J Phys Chem Solids 59:1241
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rambabu, U., Munirathnam, N.R., Prakash, T.L. et al. Synthesis and characterization of morphologically different high purity gallium oxide nanopowders. J Mater Sci 42, 9262–9266 (2007). https://doi.org/10.1007/s10853-007-1869-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1869-2