Abstract
The inhibiting action of alkyltriphenylphosphonium iodine salt ((C8H17)Ph3P+,I−) towards the corrosion behaviour of nickel in 1 M H2SO4 solution has been studied. This compound was found to retard both anodic and cathodic reactions of nickel corrosion. At constant temperature, the corrosion rate decreases with increasing inhibitor concentration. On the other hand, the increase in temperature leads to an increase in the corrosion rate. The activation energy, ΔE a, were calculated. They were found 19.3 kJ mol−1 and 71.1 kJ mol−1, respectively for the uninhibited solution and in the presence of 10−3 M of phosphonium salt. The inhibitor adsorption was identified to occur according to Langmuir isotherm. The equilibrium constant, k, as well as the free energy of adsorption, Δads G°, for inhibitor process were then calculated. Phosphonium iodine exhibited a singular behaviour for T ≥ 318 K where inhibitor desorption increases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphonium salts are considered as excellent corrosion inhibitor, especially in acidic media. In fact, Morad [1] used six organic phosphonium compounds of the structure YTP+,X−, (T= phenyl, X = Br− or Cl− and Y = propyl, propargyl, cyclopropyl, allyl, 1,3- dioxolanyl and cinnamyl) to inhibit the corrosion of mild steel in aerated 0.5 M H2SO4 solution. Khaled [2] evaluated the inhibiting action of (chloromethyl) triphenyl phosphonium chloride, triphenyl (phenylmethyl) phosphonium chloride and tetraphenyl phosphonium chloride on the corrosion of iron in 1 M HCl solution. In previous works [3], we tested tetraphenyl phosphonium bromide as nickel corrosion inhibitor in sulfuric acid medium. Recently, we have evaluated the effect of R+,X− (R+ = (C8H17)Ph3P+ or K+, X− = I− or Br− or Cl−) salts addition on the corrosion behaviour of nickel in 1 M H2SO4 medium [4]. The results achieved allowed us considering that phosphonium iodine addition modifies the interface behaviour due to the interaction between the molecule and the material surface.
Thus, in this research program we studied phosphonium iodine salt adsorption on nickel when immersed in 1 M sulphuric acid medium.
Experimental
Electrochemical experiments were conducted in a classical three-electrode cell. Saturated calomel electrode was used as reference and a platinum wire as counter electrode.
Working electrodes were prepared from high purity nickel (>99.99 wt%). Before use, they were embedded in a chemically inert resin and mechanically polishing up to 2500 SiC grade. Their active area is equal to 1 cm2.
An aerated 1 M H2SO4 aqueous solution, prepared from ultra pure reagent, was used as corrosive electrolyte. The halide salt was C8H17Ph3P+,I− and it was prepared in the laboratory by adding triphenylphosphine to heptane iodine.
The electrochemical instrumentation consisted of a Taccussel potentiostat-galvanostat PGP 201. Voltamaster 1 software was employed for instrumentation control and data treatment.
Results and discussion
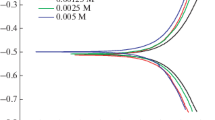
Figure 1 shows anodic and cathodic polarization curves for nickel electrode immersed for 1 h at open circuit potential in 1 M sulphuric acid aqueous media, in the absence and presence of 10−3 M of the iodine alkyl triphenyl phosphonium. The scan rate has been fixed at 8.33 mV/s and the temperature at 298 K.
In absence of the halide salt and independently on the temperature, one notes the presence of four regions on the polarization curves. The region I represent the cathodic domain characterized by a charge transfer process. It can be assigned to the strong concentration of acid (1 M) and the mobility of the H+ ion that conceals the contribution of the oxygen in the global kinetics of the cathodic reaction. In the region II the electrochemical process at the Nickel–H2SO4 interface is controlled by activation kinetic. The extrapolation of this part to the potential of corrosion permits us calculating the material corrosion rate. The region III is characterized by the presence of two oxidation peaks (1a) and (2a) corresponding to the electrochemical generation of nickel oxides and/or sulfates. Then, the current falls (region IV). A surface steady state appeared indicating that the corrosion products formed at the electrode surface allowed a pseudo-passivation of the material.
When the inhibitor is added, we observed an increase of the cathodic current density especially for region I. Then, the current density tends to a plateau. Thus, the cathodic reaction is limited by oxygen diffusion. Finally, the current density increases corresponding to H+ reduction.
One can conclude that the organic halide salt denotes an inhibition activity for the cathodic reaction taking place at the surface of nickel in 1 M H2SO4.
For the anodic domains (regions II, III and IV), one can notes that the corrosion potential tends to more anodic value. Indeed, only the second activation peak is observed. Furthermore, the steady state current decreased for the entire over potential interval considered.
Hence, one can conclude that organic iodine salt acts as mixed inhibitor. The observed inhibitive action could be due to an adsorption phenomenon at the surface of nickel making a barrier for charge and mass transfer between the metal and its environment.
To characterize the nickel interfacial behaviour, we carried out the voltammetric investigation at various temperatures ranging from 308 K to 328 K. The polarization curves obtained are reported in Fig. 2.
We have measured the corrosion potential (E corr) and the corrosion current densities (j corr) from the points of intersection of the extrapolated anodic and cathodic Tafel regions. Then, the Inhibition Efficiency percentage (IE%) and the degree of surface coverage (θ) were calculated as follow:
where j 0 and j 1 are the corrosion current densities obtained in the absence and the presence of the inhibitor.
The results obtained are gathered in Table 1.
From the data of Table 1, the following conclusions can be made:
-
(1)
at constant temperature, IE% increases with the increase in the concentration of the studied inhibitor. Such behaviour could be explained on the basis of increased adsorption of the inhibitor on the metal surface;
-
(2)
in the presence of the same concentration of each inhibitor, IE% decreases with the rise in temperature due to the enhanced effect of temperature on the dissolution process of nickel in H2SO4 solution and; or the partial desorption of the inhibitor from the metal surface.
The dependence of the corrosion rate on temperature can be expressed by the Arrhenius equation:
where:
-
ΔE a: the activation energy of the corrosion reaction;
-
T: the absolute temperature;
-
R: the universal gas constant.
Figure 3 shows the Arrhenius plots for the corrosion rate of nickel in 1 M H2SO4 with and without the presence of 10−3 M of phosphonium iodine slat.
The results give rise to satisfactory straight lines from the slopes of which one can calculate the activation energy. ΔE a values were found to be 19.3 kJ mol−1 for the uninhibited solution and 71.1 kJ mol−1 in the presence of 10−3 M of phosphonium salt. The value of ΔE a for the inhibited solution is higher than that for uninhibited one, indicating the greater tendency of such compound to react at the surface of nickel, in 1 M H2SO4 electrolyte [5–7].
From the above mentioned results it is concluded that the inhibition of nickel corrosion in 1 M H2SO4 solution occurs by adsorption of the additive used [8, 9].
The most commonly used isotherms for studying the adsorption mechanism of an inhibitor on a metal electrode surface are listed in the literature [10–19].
In order to choose the appropriate isotherm model to describe the adsorption mechanism of phosphonium iodine at nickel surface, we plotted C/θ versus concentration C (Fig. 4).
A linear relationship is obtained, independently on the temperature considered. To explain such result, we considered that the surface coverage (θ) of the inhibitor on the nickel specimen is related to the concentration (C) of the inhibitor in the bulk of the solution according to Langmuir adsorption isotherm:
where k is the equilibrium constant for the adsorption process.
Rearranging Eq. 4:
One can conclude that the absorption of phosphonium iodine salt on nickel surface in 1 M sulphuric acid medium occurs according to the Langmuir adsorption isotherm.
From the intercepts of the straight lines on the C/θ axis, k values could be calculated (Table 2).
Furthermore, the equilibrium constant (k) for the adsorption process of the inhibitor could also be related to the free energy of adsorption Δads G° by the relationship:
The calculated values are gathered in Table 2. Then, we plotted Δads G° against temperature (Fig. 5).
Independently on the temperature considered, negative values are obtained for Δads G° indicating the spontaneous interaction between phosphonium iodine and nickel surface.
However, the free energy decreases from −28.8 kJ mol−1 at 298 K to −33.6 kJ mol−1 at 318 K. A linear relationship could be used to describe Δads G° variation versus T in this interval. Then, other thermodynamic functions can be calculated through the following equation:
where Δads H° and Δads S° the enthalpy and the entropy of adsorption. They were equal to +43.4 kJ mol−1 and +240 J K−1 mol−1, respectively.
In the temperature domain considered, both thermodynamic functions values are positive reflecting for phosphonium iodine adsorption on nickel surface:
-
(1)
an endothermic behaviour;
-
(2)
an increase of the molecular disorder.
Then, the free energy exhibited a singular behaviour. In fact, it increases reaching −28.4 kJ mol−1. Such result could be attributed to the competition adsorption/desorption of the additive on nickel surface for T ≥ 318 K.
Conclusion
The results of these experiments revealed that:
-
(1)
phosphonium iodine decreases the corrosion rate of nickel in 1 M H2SO4 electrolyte;
-
(2)
at constant temperature, IE% increases with increasing inhibitor concentration;
-
(3)
at the same concentration, IE% decreases with increasing temperature;
-
(3)
inhibition occurs by adsorption according to the Langmuir isotherm;
-
(4)
inhibitor desorption increases for T ≥ 318 K.
References
Morad MS (2000) Corros Sci 42:1307
Khaled KF (2004) Appl Surf Sci 230:307
Niass SO, Touhami ME, Hajjaji N, Srhiri A, Takenouti H (2001) J Appl Electrochem 31:85
Said F, Souissi N, Dermaj A, Hajjaji N, Triki E, Srhiri A (2005) Mater Corrosion 56(9):619
Saleh JM, Al Haidari YK (1989) Bull Chem Soc Jpn 62:1237
Abd El Nabey BA, Kamis E, Ramadan MS, El Gindy A (1996) Corrosion 52:671
Abd El Aal EE, Zakria W, Diab A, Abd El Haleem SM (1999) J Chem Technol Biotechnol 74:1061
Dinnappa RK, Mayanna SM (1982) Corrosion 38:525
Rudresh HB, Mayanna SM (1977) Surf Technol 6:139
Langmuir I (1918) J Am Chem Soc 40:1361
Frumkin AN (1925) Z Phys Chem 116:466
Hill TL (1952) J Chem Phys 20:141
de Boer JH (1953) In: The dynamical character of adsorption. Oxford University Press, Oxford
Parsons R (1964) J Electroanal Chem 8:93
Damaskin BB, Petrii OA, Batrakov VV (1971) In: Adsorption of organic compounds on electrodes. Plenum Press, New York, p 86, 94 and 247
Kastening B, Holleck L (1965) Talanta 12:1259
Bockris JO_M, Swinkels DAJ (1964) J Electrochem Soc 111:736
El-Awady AA, Abd-El-Nabey BA, Aziz SG (1992) J Electrochem Soc 139:2149
Khamis E, Hosny A, EL-Hadary S. (1995) Affinidad 95:456
Acknowledgements
The authors would like to thank AUF (Agence Universitaire de la Francophonie) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Said, F., Souissi, N., Es-Salah, K. et al. Phosphonium iodine as nickel corrosion inhibitor in 1 M sulfuric acid medium. J Mater Sci 42, 9070–9074 (2007). https://doi.org/10.1007/s10853-007-1640-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1640-8