Abstract
An intensive study has been undertaken on the damping mechanism of chlorinated butyl rubber (CIIR) and phenolic resin (PR) vulcanized blends. Evidence from morphology observations of blend sections by scanning electron microscopy, extraction experiments, molecular weight analysis by gel permeation chromatography indicates that except a part of PR crosslinked with CIIR, the others remain linear and disperse in the CIIR matrix to form a two-phase continuity. The tan δ curves of blend without striking peak split from the dynamic mechanical spectra and inward shift of glass transition of linear PR suggests that cured CIIR and linear PR is miscible to a certain extent, thus, the phase separation in the blends can be described as slight phase separation. There observations are interpreted in terms of variations of free volume characterized by positron annihilation lifetime spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Butyl rubber (IIR), a copolymer of isobutene and isoprene (usually 3%), is well-known for its strong capability of energy dissipating combined with good airtightness, ozone resistance and chemical stability [1]. With the application of polymeric damping material in civil products, IIR has received increasing interests chlorinated butyl rubber (CIIR), halogenated derivative of IIR, is widely applied instead of IIR because of its high vulcanization speed and good compatibility with other rubbers besides the heritage of the excellent properties of IIR [2].
The damping property of polymer is generally governed by its behavior of transition or relaxation, which are dominated by its components and structures [3]. Figure 1 [4] shows that the tan δ peak of CIIR is pretty wide covering a temperature range of −62 °C to 11 °C (tan δ ≥ 0.3) with a maximum at −28 °C and a shoulder peak near −53 °C Among it, the low-temperature shoulder peak corresponds to the T g of CIIR, while the transition above T g is designated as “liquid-liquid” transition (T II) by Boyer [5], who proposed that T II is related to the disruption of short-range order, but the precise mechanism is not clear yet. It is undoubted that T II is very important for damping properties of vulcanizate of CIIR. Due to the unique T II of CIIR the loss peak is broad and high, however, because its highest peak appears at low temperature zone, CIIR utilized as damping material at room temperature is often limited.
Considerable researches have focused on extending the damping functional region of CIIR towards high temperature area. There are several main approaches, such as dynamically vulcanization, IPNs, blend of CIIR and resin, to name just a few.
Liao [6] and co-workers prepared a series of dynamically cured butyl rubber/polypropylene blends, whose loss peaks of the blends tend to shift towards high temperature. However, tan δmax was not over 0.35, since the T II of butyl rubber was suppressed by polypropylene. He [7] and Huang prepared two kinds of CIIR/PMAC interpenetration networks, among which CIIR/PEA IPNs exhibited a high damping value with tan δmax ≥ 1.24 at 10 Hz , while CIIR/PBMA IPNs exhibited a wide damping zone of −45 °C to +57 °C (tan δ ≥ 0.3 ). Luo [8] and co-workers researched the effect of blend ratio and curing system on the dynamic mechanical properties of CIIR/IIR blends, whose results showed that both storage modulus (E′), loss modulus (E′′) and tan δ rise when the amount of PR increases in the blends but T g of blends slightly moved to high temperature. Luo’s research revealed that T g of CIIR shifted towards low temperature although adding plasticizer agent can increase tan δ. Obviously, it is not an effective method to extend the transition zone to high temperature area. Even though every approach mentioned above can improve the damping property of CIIR to different extent, each of them has some drawbacks.
The research of Dutta [9] and Tripathy indicated that the dynamic mechanical properties of CIIR depended significantly on the concentration of the phenolic curative. CIIR can be vulcanizated by use of phenol-formaldehyde resins (resols) besides the conventional sulfur curatives [10]. Phenolic resin (PR) occurs reactions with CIIR via double bond in CIIR provided by isoprenes. The dynamic mechanical properties of the vulcanized CIIR/PR blends have been discussed in the literatures by Pan [11], Chen [12] and Ding [13], whose researches all indicate that this method can remarkably shift the transition area of CIIR towards high temperature and broaden the transition zone of CIIR in order to satisfy the application at room temperature. Thus, the viscoelastic damping material based on the cured blend of CIIR and PR has been used for anti-vibration in the field of aviation as well as for acoustic attenuation in civil products [14]. However, the damping mechanism of such material was not clear yet until today, and there are few literatures concerning the subject that can be found. Therefore, it is pronounced that to clarify why PR could broaden the transition region of CIIR to high temperature is significant whether for better understanding the phenomenon or for well preparing the materials.
Positron annihilation technique (PAT) is an effective method of measuring free volumes of materials and getting the information of microstructure via interaction between positrons and materials [15]. The beginning utilization of PAT was in the metal and semiconductor material. In 1972, when Tao S J proposed an equation after doing a great many researches on the relationship of the free-volume hole and positron annihilation lifetime [16]. Recently positron annihilation lifetime spectroscopy has been used as a unique and potent method for characterizing the free volumes and the transitions of polymer [17].
In the present paper, we intensively investigated the damping mechanism of the vulcanized blends of CIIR with PR. A novel interesting result is obtained by scanning electron microscopy (SEM), gel permeation chromatography (GPC), dynamic mechanical analysis (DMA) and positron annihilation lifetime spectroscopy (PALS).
Experimental
Material
The CIIR (Exxon 1068) was from Exxon Co., USA. The PR (201 resin) was from Chongqing Resin Factory, China.
Sample preparation
A laboratory-scale external mixer equipped with a pair of roller blades was used for blending. Blends of CIIR with different PR content (5–50 phr) were prepared via mechanical mixing at room temperature for 6 min. The cured blends were compression moulded (20 min at 160 °C) to form sheets 4mm in thickness. The composition for the moulded samples is summarized in Table 1, and the concentration of ZnO, SA, MgO in all the samples are 5 phr, 1 phr and 0.5 phr, respectively.
Dynamic mechanical analysis
The samples were trimmed to dimensions of 10 mm × 12.5 mm × 4 mm. Dynamic mechanical analysis was conducted on TA Q800 (TA company, USA) analyzer under a heating rate of 2 °C min−1 within a temperature range of −100 °C to 80 °C and a frequency of 10 Hz for temperature scanning.
Extraction experiment
Soxhlet extraction (in acetone) was used to determine the PR content crosslinked with CIIR. Specimens of blends about 1 g were cut into thin slices, dipped them into solvent of acetone and extracted for 72 h under ambient temperature. After extraction, the blends were vacuum-dried overnight at 70 °C, and then the extractive can be measured.
Scanning electron microscopy analysis
Preparation of sample for observation of morphology with SEM (JSM-5900LV, Japan) was carried out as follows. The blend sheets were fractured in the liquid nitrogen, the fractured surface was treated with acetone for 72 h to remove the PR uncrosslinked with CIIR. After then, the surface was coated with gold by vacuum deposition.
Gel permeation chromatography analysis
The molecular weight of extractive is measured with GPC(AGILENT-1100, USA) for comparison with original PR (Elution solvent: THF; temperature: 25 °C).
Differential scanning calorimetry
Extractive after removing the solvent was analyzed with a DSC 204 (NETZSCH, Germany), at a heating rate of 20 °C min−1 under a constant nitrogen flow. Glass transitions were taken as the mid-point of the inflexion.
Positron annihilation lifetime spectroscopy (PALS) analysis
In this paper, the PALS measurement (ORTEC, USA) were performed using a conventional fast-fast coincidence system which has a time resolution of 240 ps with energy window set for 60Co. The positron annihilation lifetime was measured at room temperature (20 °C).
Result and discussion
Dynamic mechanical properties of cured CIIR/PR blends
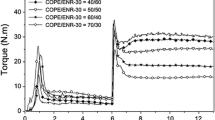
Figures 2 and 3 show the curves of tan δ and storage modulus (E′) versus temperature with different PR content and damping properties of cured blends are listed in detail in Table 2. It can be observed from Fig. 2 that the low temperature shoulder peaks are related to T g of CIIR, as also indicated by the corresponding low-temperature drop of E′ in this temperature range as shown in Fig. 3, and the maximum peaks at high temperature correspond to T II of CIIR. Dynamic mechanical properties of the blends are influenced significantly by the amount of PR, and here the behaviors of tan δ curved are of great interest. With increasing the PR content, the broader loss peaks are obtained, while T g of CIIR and especially T II shift towards higher temperature, making the latter be more clearly discerned. Simultaneously, it can be seen from the E′ curves the high-temperature inflexions are more obvious, which is related to T g of PR in the blends. Because PR occurs reaction with double bonds in CIIR provided by isoprenes, the content PR reacting with CIIR is certain for the fixed amount of CIIR. In that case, what a form of extra PR exists in the blends and what effect of superfluous PR takes in the blends after the CIIR reaches saturation in crosslinking degree is really important and pretty worth investigating for studying the variation of transition peak.
The linear PR and its behaviors in cured CIIR/PR blends
The mechanism of PR vulcanization of butyl rubber has been studied by some scientists, among which Greth’s chroman mechanism of resin cure is widely accepted [18], which is depicted below as following. The first step PR is envisioned to be a dehydration to give an o-methylene quinone intermadiate, and then this combines with a rubber double bond in a Diels–Alder type reaction to give a product with the chroman structure. It is the second addition reaction that gives the crosslinked product. According to Greth’s mechanism, PR content of requirement to vulcanizate CIIR is certain, which means that PR exists in blends with two different structures, namely crosslinking form and linear one.
What should be noted is that the linear PR can be dissolved in acetone, however, it no longer dissolves in the same solvent provided it crosslinks with butyl rubber, therefore, acetone can be used for extracting the uncrosslinked PR in the cured blends.
After extraction it can be found that a large amount of uncrosslinked PR was dissolved in acetone. At the same times, the morphological feature of the resulting samples after extraction was observed with SEM as shown in Fig. 4. The blend with PR content of B-01 (Fig. 4a) displays a single-phase morphology, whereas the blends of B-04 and B-08 (Fig. 4b, c) exhibit dual-phase morphology with PR domains dispersed in the CIIR matrix. The dimension of PR domains of B-04 and B-08 are 9.52 μm and 19.00 μm, respectively, indicating that PR of B-01 appears to be completely crosslinked with CIIR to form a single phase, and more linear PR exists in the cured blends with an increase in PR content. Table 3 shows the extractive content of CIIR with different PR content, where the extractive content was measured, and normalized with respect to100 phr CIIR. After repeating experiments and calculations according to equation as follow, it can be derived that the saturated amount of PR crosslinked with 100 phr CIIR is equal to 20 phr.
where M is PR content (phr) crosslinked with 100 phr CIIR, M PR and f refer to PR content (phr) and corresponding extractives content respectively as listed in Table 3.
In order to ascertain the structure of the extractives, the molecular weight of original PR and extractives were examined with GPC. The molecular weight and its distribution of original and extractives PR are shown in Fig. 5. It can be gained that the number average molecular weight of original PR is 1,575 g mol−1, but only 750 g mol−1 for extractives, hence the extractive is oligomer based on the reserved time in the chromatographic column. This may be attributed to that PR degraded to different extent. In the blending process, the system presents alkaline owing to the use of ZnO and MgO as curing accelerant. So in such alkaline condition the linear PR probably degraded at cure temperature [19].
Therefore it is believed that in the blending system a part of PR crosslinked with CIIR, and the rest was blended with crosslinked CIIR. Consequently, PR played two roles of vulcanized agent and another blending component in the vulcanized blend. Further, T g of the linear PR extracted from the blend was measured by DSC. From DSC curve shown in Fig. 6, it can be seen that T g of linear PR is 14.87 °C.
The damping mechanism of cured CIIR/PR blends
The correlation of PR content dependence on the longest lifetime τ3 and its intensity I 3 are shown in Figs. 7 and 8, respectively. Each position annihilation lifetime spectroscopy was resolved into three component using PATFIT program, and the variances of fit are smaller than 1.15. In the paper, aimed at finding a relation between the dynamic mechanical behaviors of the blends and the free volume properties we focus on the longest lifetime (τ3) and its intensity (I 3). The longest component (τ3 ≈ 0.8 ∼ 3.5 ns) is attributed to o-Ps pick-off, annihilating in the free-volume holes of amorphous regions and its intensity (I3) is related to the quantity of free volume in the regions where positions are introduced [20]. The o-Ps lifetime exhibits a general decrease tendency as a function of PR content up to 50 phr. On the other hand, the intensity I 3 shows a sharp drop with increasing PR content up to 20 phr, and then keeps constant to 45 phr, after that goes down. This is due to the fact that when PR content is less than 20 phr, PR crosslinks totally with CIIR and a single-phase morphological feature can be gained. The crosslinking density of the blend increases with ascending in PR content, as a result the size and quantity of free-volume hole are abated, which causes an effect of the rise in energy barrier of segment motion, so T II moves to high temperature. This is consistent with the variation of tan δ curves estimated by DMA.
When PR content is more than 20 phr, from the morphology analysis it can be considered that the linear PR and cured CIIR exhibit dual-phase structure. With PR amount going up from 25 phr to 50 phr, the dimension of PR phase zone increase from 9.52 μm to 19 μm, which means that the degree of phase separation aggrandizes. These morphological features are in accord with the evidence of DMA that the high-temperature inflexions tend to be more and more apparent.
Although with an increase in linear PR phase separation of blends emerges, there is no striking split of peak in tan δ curves, which is an interesting phenomenon especially worth noting. Comparing to T g of linear PR at 14.87 °C, it can be observed that the T g of linear PR corresponding to high-temperature inflexion in the storage modulus curves move towards below 0 °C. Such inward shifts of linear PR glass transition means that cured CIIR and linear PR are miscible to some extent. In order to investigate such phenomenon the size of free volume and its density must be discussed. The reduction in free volume of blends after PR content reaches 20 phr indicates that the presence of linear PR further limits segment motion of vulcanized CIIR. Since the structure of octyl group in PR is similar to butyl group in CIIR, there must be some interaction between the two groups, we defined which as intermolecular nonbonding complexation. The sketch of relationship between CIIR and PR is shown in Fig. 9. PR crosslinks with CIIR to form the chemical crosslinking, and the other linear PR disperses in the vulcanized CIIR aggregates and nonbonding complexation between linear PR and CIIR comes into being. The formation of nonbonding complexation in the blends induces that this is not completely phase separation between linear PR and cured CIIR. In addition, PR crosslinked with CIIR play a role as a compatilizer between linear PR and cured CIIR. Therefore, linear PR is partially miscible with CIIR. This conclusion well interprets why tan δ curves are broadened without split of peak.
On the other hand, with content of linear PR in the vulcanized blends the quantity of nonbonding complexation goes up, chain motion of cured CIIR is astricted further, in that case, the relaxation of chain requires more energy, which results in that tan δ curves measured by DMA move towards high temperature and the value of tan δmax minishes. This is can be considered that the presence of linear PR suppresses T II.
Conclusions
In present paper, the damping mechanism of CIIR and PR vulcanized blends was investigated. It could be obtained that the saturated amount of PR crosslinked with 100 phr CIIR is 20 phr. The superfluous PR remained linear and dispersed in the CIIR matrix to form a dual-phase continuity. And the high-temperature inflexions in storage moduls curves also confirmed this.
The evidence from the tan δ curves without split of peaks and the inward shift of T g of linear PR proved that cured CIIR and linear PR were miscible to some extent, which could be explained in term of the variation of free volume. Because of the same structure of octyl group in PR and butyl group in CIIR intermolecular nonbonding complexation forms in the blends, furthermore, PR crosslinked with CIIR play a role as a compatilizer between linear PR and cured CIIR. Accordingly, this phase separation can be considered as a slight phase separation, and it is enough to make the tan δ curves be broadened with no obvious peak split.
In addition, both crosslinked PR and linear PR in blends make free volume of the blends decrease, so tan δ curves shift to higher temperature and the value of tan δmax minishes, which proves that T II is restricted.
References
Brydson JA (1988) Rubber material and their properties, vol 8. Elsevier, London, p 1954
Houston, Texas (1998) Exxon Chem 6
Wu CF (2001) J Appl Polym Sci 80:2468
Liao FS, Su AC (1994) Polymer 35(12):3579
Boyer RF (1987) In: Keinath SE, Miller RL, Rieke JK (eds) Order in the amorphous state of polymer. Plenum, New York, p 135
Liao FS, Su AC (1994) Polymer 35(12):3579
He XR, Huang GS (2005) Chinese J Appl Chem 22(4):382
Luo QK (2004) China Synthtic Rubber Industry 27(1):26
Dutta NK, Tripathy DK (1990) Polym Degrad Stab 30:231
Capps RN (1986) Rubber Chem Technol 59:103
Pan J (1989) Aerospace Mater Technol 3:53
Chen BY, Ma GF (2005) World Rubber Industry 32(2):31
Ding GF, Wang JH (2004) Rubber Industry 51:571
Zhao YF, (2001) Aerospace Mater Technol 2:19
Hsieh TT, Tiu C (2000) Polymer 41:4737
Nakanishi H, Wang SJ, Jean YC (1988) In: Sharma SC (ed) Positron annihilation studies of fluids. Singapore, World Science; Singapore, p 292
Jean YC (1990) Macrochem J 42:72
Robert PL, Robert AK, Robert W (1989) Layer Rubber Chem Technol 62:10
Pritchard G (1980) Developments in Reinforced Plastics-I: Resin Matrix Aspects, Essex: Appl. Science Pub
Li HL, Ujihira Y, Nanasawa A, Jean YC (1999) Polymer 40:349
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (Grant No. 10276025) and the Ministry of Education (the Foundation for Ph.D. training, Grant No. 20040610027) of China.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Qu, L., Huang, G., Wu, J. et al. Damping mechanism of chlorobutyl rubber and phenolic resin vulcanized blends. J Mater Sci 42, 7256–7262 (2007). https://doi.org/10.1007/s10853-006-1466-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1466-9