Abstract
The magnetic properties and hardness of a Ni–Co–Mo–Ti maraging steel 300 grade were measured as function of aging temperature. The austenite and martensite phase quantifications in the different heat treatment conditions were carried out by X-ray diffraction using direct comparison method. The behavior of the hardening, magnetization saturation and coercive force against aging temperature and time were explained taking into account the variation of austenite volume fraction with aging time and temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maraging steels are low-carbon martensitic steel developed for high-performance applications as in ultra-high speed rotors for electric hysteresis motors. For this purpose, ultra-high strength is required along with good dimensional stability during heat treatment in combination with appropriate magnetic properties. According to Belozerov [1], the increase of the rotational speed of the disk rotors to about 105 rpm required a yield strength to at least 1300–1500 MPa. The coercive force (H c) must be low, the saturation (B s) and residual induction (B r) in fields as low 20–60 A/cm must be high and the squareness ratio (B r/B s) must be at least 0.7.
In the solution treated condition the maraging steels presents a soft and deformable martensitic structure, with hardness values between 280 and 320 HV [2]. These properties are attributed to the low carbon and the high nickel content in the martensite.
The maraging steels are age-hardenable in the 400–650 °C temperature range. The aging in temperature below 450 °C produces ordered and coherent phases (μ, S and/our ϖ) in the martensitic matrix. The aging between 450 and 600 °C is rapid and intense, and it is attributed to the precipitation of the intermetalics compounds Ni3(Mo,Ti) and Fe2Mo in the dislocations [2–4]. Aging between 500 and 700 °C promotes the austenite formation by a diffusion-controlled decomposition reaction: α1→α2 + γ, where α1 is martensite, α2 is a low nickel bcc phase and γ, is the nickel enriched austenite phase [5, 6]. According to Li [7] the austenite formation occurs at the same time and as consequence of the partial dissolution of the Ni3(Ti, Mo) and the precipitation of Fe2Mo. The austenite phase formed in high temperatures is total or partially retained at the room temperature, depending on its nickel content.
In this current work, was measured the magnetic properties of an 18%Ni maraging steel with Co, Mo and Ti as function of aging temperature in the 440–650 °C range. Two initial conditions were compared: solution treated and cold deformed. The aging time effect was also evaluated in the temperatures of 440, 510, 560, 600 and 650 °C. X-ray diffraction quantifications by the direct comparison method have been determined in various treatment conditions to evaluate the austenite influence in the hardness and magnetic properties.
Experimental
The material chosen for the research was a 20 mm × 10 mm × 2.5 mm sheet samples of maraging 300 steel, whose composition is given in Table 1. Specimens were solution treated at 900 °C ± 10 °C for 40 min, followed by cold rolling with true deformations 0.85 and 1.61. Some samples were solution treated and aged, and others were aged without solution treatment after deformation. The samples were aged at 440, 480, 510, 560, 600 and 650 °C for different treatment times.
In all samples of each aging temperature and time conditions 30 kgf Vickers hardness tests were performed.
X-ray diffraction was carried out in a PHILIPS® diffractometer, model X’Pert Pro, in step scan mode with step size of 0.02°, time per step of 3 s and angular interval 45–125°. Radiation CoKα (1.7890 Å) was used with 40 kV and 40 mA. The volume fraction of the austenite (γ) and martensite (α) phases were obtained by the direct comparison method, described by Cullity [8], and taking into account the different values in the scattering factors for austenite (γ) and martensite (α) phases for maraging, as steels suggested by Pardal et al. [9].
The magnetic properties were measured in a vibrating sample magnetometer (VSM) using disk shaped samples with 3.5 mm of diameter. The magnetic measurements were taken at room temperature with maximum applied field of 5 kOe (400 kA/s), time constant 1 ms and total measure time of 30 min. The data were corrected for demagnetization fields using the suggested by Chikazumi equations [10].
Results and discussion
Figure 1 shows the precipitation hardening curves of the maraging 300 steel aged at 440, 480, 510 and 560 °C.
The 440 and 480 °C curves had not shown overaging until 24 h. The maximum hardness for these two temperatures was obtained for 24 h (637 HV) and 10 h (648 HV), respectively. The samples aged at temperature 510 °C for different times presented a maximum peak hardness of 629 HV after 4 h of aging, and according to Figure 1, aging times higher than 10 h produce a slightly reduction in the hardness. In the aging at 560 °C the hardness peak (601 HV) occurs at 1 h, and overaging is observed after this time.
The aging at low temperatures (440 and 480 °C) shows that for short times the hardness increases slowly and continuously, in comparison with aging at higher temperatures. Initially the age hardening is due to the formation of S precipitates, that give place later to the ordered ω precipitate as the reported by many researches [2–4, 11].
For aging at 510 °C the hardness peak is due to the precipitation of ellipsoidal Ni3(Ti, Mo) at the dislocations clusters in the martensite matrix [2, 4]. In the case of aging at 560 °C, this phenomenon occurs faster. The overaging, mainly at 560 °C, is due to Fe2Mo precipitation which maintain the hardness in a higher level for longer aging times [12]. One of the reasons for the hardness reduction, however, beyond the precipitates growth, is the partial dissolution of the Ni3(Ti,Mo) intermetallic compound which gives place to the formation of the above mentioned Fe2Mo and the nickel rich austenite [12].
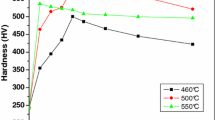
Figure 2 shows the precipitation hardening curves of the maraging 300 steel at 560, 600 and 650 °C. In this figure, for the samples treated at the 600 and 650 °C, the hardness peak occurs for 15 min of aging, and presents values of 567 and 465 HV, respectively.
In the overaged samples, the hardness decreases due to the austenite precipitation. The growth, coalescence and incoherence of the precipitates also influence the drop of this property. Figure 3 presents the influence of the austenite volumetric percentage in the hardness for 560, 600 and 650 °C in different aging times. In this figure, it can be observed the increase of the precipitated austenite produces a substantial drop in the hardness for the aged samples at 560 and 600 °C in different time conditions due to dissolution of intermetallic precipitates. Minimum hardness value is obtained in the sample aged 650 °C for 24 h. At 650 °C, in contrast with 560 and 600 °C, the amount of austenite decreases with the increase of aging time after 1 h.
The saturation magnetization (m s) and the coercive force (H c) variations with aging temperature are shown in Figs. 4 and 5, respectively. In this figure, the samples were aged for 1 h. The aging between 560 and 700 °C produces more intense variations in the m s and H c values, which is attributed to the nickel rich reverted austenite formation. The minimum value of m s (88 A2 m/kg) and the maximum values of H c (19.83 kA/m) are both obtained in the sample aged at 650 °C for 1 h, in agreement with results obtained by Tavares et al. [13] in the maraging 350 steel.
Figure 6 shows the squareness ratio values (B r/B s) as function of the aging temperature, in solution treated and aging samples during 1 h. In samples aged between 520 and 600 °C the squareness ratio values are higher than 0.8, which is characteristic in anisotropic materials for applications in electric rotors. However, B r/B s values smaller than 0.7 were found in samples aged at 650 and 750 °C.
The behavior of the coercive force (H c) against the aging time is shown in the Fig. 7. A small effect of magnetic hardening (increase of the coercive force) was observed at 510 °C, but not at 440 °C. Therefore, it can be concluded that the mechanical hardening effect is more important than the magnetic hardening in these two temperatures. During the aging at 560 °C a small magnetic hardening is produced, despite of the small hardness decrease with the aging time after 1 h. In the samples aged at 600 °C the H c undergoes an important increase in the first hour of treatment, due to intense austenite precipitation. The aging at 650 °C for 1 h promotes the maximum austenite precipitation and, as consequence, H c is maximum and m s is minimum. However, H c presents a small increase with the aging time after 1 h. In agreement, Fig. 8 shows that, after the drop in the first hour of aging at 650 °C, m s starts to increase. These facts are related to the behavior shown in Fig. 9. The austenite volume fraction measured at room temperature increases with aging time at 560 and 600 °C, but decreases after 1 h at 650 °C. The reasons for the decrease of austenite content at 650 °C are explained in another reference [9].
A correlation between the magnetization saturation (m s) and the austenite volume fraction measured by XRD was searched in order to obtain a magnetic method for austenite quantification in the maraging 300 steel. Magnetic phase quantification is successfully used to quantify α′ martensite in deformed austenitic stainless steels [14], ferrite phase in duplex [15] and superduplex stainless steels, austenite phase in TRIP steels [16]. In these examples, a linear correlation between m s and the magnetic phase volume fraction was obtained, since the intrinsic magnetic saturation of the ferromagnetic phase was almost constant. In maraging steels, however, the chemical compositions of martensite and austenite phases change significantly with the aging temperature and time. As consequence, the intrinsic magnetization saturation of the ferromagnetic phase (martensite) is strongly affected by the aging conditions. This explains why the m s versus austenite content (%) curve (Fig. 10) could not be fitted by a straight line. Alternatively, the data presented in Fig. 10 were fitted by a square function, as shown in Fig. 11. The fitted equation can be used to obtain an estimative of the austenite content in the maraging steel aged between 500 and 650 °C.
where: γ—Austenitic volumetric percentage, m s—magnetization saturation (emu/g or Am2/kg).
Conclusions
The aging at 560 °C for 1 h allows high mechanical resistance and good magnetic properties (B r/B s,H c,m s) in the maraging steel 300. At this aging condition, a small amount of reverse austenite is formed and retained at room temperature, but there was no significant effect on the magnetic properties and hardness.
The increase of the austenite content above 560 °C promotes the decrease of the saturation magnetization (m s) and hardness, concomitant with the decrease of coercive force (H c). Increasing the aging time at 560 and 600 °C also raises H c and makes m s falls. On the other hand, at 650 °C, the increase of aging time promotes the decrease of the austenite content, which induces the increase of m s and the decrease of H c.
A square function was adjusted to obtain a correlation between the saturation magnetization (m s) and the amount of austenite. This correlation can be used to obtain an estimative of the austenite volume fraction in maraging steels.
References
Belozerov EV, Sagaradze VV, Popov AG, Pastukhov AM, Pecherkina NL (1995) Phys Metal Metall 79(6):606
Magnée A, Drapier JM, Dumont J, Coutsouradis D, Habraken L (1974) Centre d’Information du Cobalt, Brussels
Lecomte JB, Servant C, Cizeron G (1985) J Mater Sci 20:3339
Tewari R, Mazumder S, Batra IS, Dey GK, Banerjee S (2000) Acta Mater 48:1187
Peters DT (1968) Trans ASM 61:62
Habiby F, Ul Haq A, Khan AQ (1992) Mater Design 13:259
Li X, Yin Z (1995) Mater Lett 24:239
Cullity BD (1956) Elements of X-ray diffraction. Addison-Wesley Publishing Company, Massachusetts, USA
Pardal JM, Tavares SSM, Cindra Fonseca MP, Abreu HFG, Silva JJM (2006) J Mater Sci. 41:4732
Chikazumi S (1964) Physics of magnetism. J. Willey, New York
Servant C, Bouzid N (1988) Acta Metall 36(10):2771
Viswanathan UK, Dey GK, Asundi MK (1993) Metal Trans 24A:2429
Tavares SSM, Da Silva MR, Neto JM, Pardal JM, Cindra Fonseca MP, Abreu HFG (2004) J Alloys Compounds 373:304
Mongonon PL, Thomas G (1970) Metall Trans 1:1587
Tavares SSM, Neto JM, Da Silva MR, Pedroza PD, Teodósio JR, Pairis S (2003) J Alloys Compounds 351(1–2):283
Zhao L, Van Dijk NH, Brück E, Sietsma J, Van Der Zwaag S (2001) Mater Sci Eng A313:145
Acknowledgements
We acknowledge Karlla M.S. Ribeiro and Muricy R. Brito from CSN/Brazil by the chemical analysis of the steel, and the Brazilian research agencies (FAPERJ and CNPq) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pardal, J.M., Tavares, S.S.M., Cindra Fonseca, M.P. et al. Influence of temperature and aging time on hardness and magnetic properties of the maraging steel grade 300. J Mater Sci 42, 2276–2281 (2007). https://doi.org/10.1007/s10853-006-1317-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1317-8