Abstract
A sol–gel procedure was used to cover the magnetic P (St-co-MAA-co-AM) microspheres with a SiO2 layer, forming MMS/SiO2 composite micospheres. The composite spheres were synthesized by a two-step process. First, magnetic P (St-co-MAA-co-MA) microspheres (MMS) were prepared by microemulsion polymerization of styrene (St), methacrylic acid (MAA) and acryamide (AM) in the presence of magnetic fluid. In the next step, MMS/SiO2 spheres were obtained by addition of tetraethyl orthsilicate (TEOS) to the mixture of MMS, ammonium hydroxide and ethanol. The morphology, structure and properties of the MMS/SiO2 were characterized and the results indicate that the MMS/SiO2 spheres are about 2 μm in size with superparamagnetic behavior. The surface of the MMS/SiO2 spheres was functioned with –COOH successfully. Bioconjugation to IgG was also studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Highly selective magnetic separation has found numerous applications in biotechnology and biomedical diagnostics [1–3]. However, Antibody-mediated isolation of cell is probably the best known practical application of magnetic particles [4]. Application in the medical realm requires magnetic particles imbedded in a nonmagnetic matrix. The nontoxic silica is an ideal coating material because of its capability to form extensive cross-linking, which leads to an inert outer shield, and the silica surface allows a variety of surface functionalities to be obtained by versatile silanation chemistry [5–7], which enables “molecular recognition” in numerous application [8–10].
In the past few decades, many methods have been developed for coating SiO2 on magnetic particles, including sol–gel [11, 12], aerosol pyrolysis [13], micelle microemulation [14] and coating the magnetic spheres with organic molecule, then directly grew silica shell [15], etc. [16]. Micron-sized magnetic spheres are the ideal material for cell separation. Spheres synthesized by microemulsion polymerization may meet the size requirement, but their surface can be only functioned with a limited special functional groups [17, 18], which limited its application realm, and large amount of functional groups were buried in the polymer with only a small part left on the surface [18].
In this study, we present a method in which magnetic P (St-co-MAA-co-MA) microspheres (MMS) were prepared by microemulsion polymerization of styrene (St), methacrylic acid (MAA) and acryamide (AM) in the presence of magnetic fluid [17]. Upon addition of precursors, silica coating would grow on the surface of the MMS spheres. Transmission Electron Microscopy (TEM), X-ray Photoelectron Spectroscopy (XPS), Fourier Transform Infrared Spectrometer (FTIR) and Vibrating Sample Magnetometer (VSM) were used to characterize the MMS/SiO2 spheres and revealed that the spheres were about 2 μm in size with superparamagnetic behavior. Then the MMS/SiO2 spheres were functioned with –COOH. Finally, the MMS/SiO2 spheres were tested for their bioconjugation ability with IgG.
Experiment and measuring methods
Experiment
Synthesis of Fe3O4 nanoparticles
Fe3O4 nanoparticles were synthesized by dropping ammonia hydroxide into a mixture of FeCl3 · 6H2O and FeCl2 · 4H2O (2:1 molar ratio) solution at 40 °C under nitrogen, then oleic acid was added as a capping agent to make Fe3O4 hydrophobic. Fe3O4 particles were precipitated and washed with ethanol and dispersed in styrene forming magnetic fluid [19].
Synthesis of magnetic P (St-co-MAA-co-AM) microspheres (MMS)
The MMS were prepared by a microemulsion polymerization process [17]. In a typical case, 0.18 g SDS and 0.08 g CA were dissolved in 50 mL water to foam up at 60 °C. Then a mixture of 6 mL magnetic fluid, 3 mL MAA, 1 mL DVB, 0.3 g BPO, 0.07 g KPS and 3.0 g AM were added. After 40 min, 150 mL water was added to the flask and allowed to polymerize at 70 °C for 3 h. The emulsion was then washed with water/ethanol and microspheres were collected.
Synthesis of MMS/SiO2 spheres
The MMS/SiO2 spheres were obtained by adding tetraethyl orthosilicate (TEOS) into a mixture of ammonium hydroxide, MMS and water/ethanol (V:V = 1/10) solution. Typically, 1.5 g MMS spheres and 6 mL ammonium hydroxide were added to 50 mL water–ethanol (1+10) solution. TEOS solution (1 mL TEOS in 49 mL anhydrous ethanol) was added drop-wise into the vigorously stirred solution. The mixture became gelatinized gradually because of the hydrolysis of TEOS. The gelatin was then kept for another 24 h to ensure maximum hydrolysis. Finally, it was dried under vacuum at 110 °C for 2 days. The dried powder was ready for the following characterization.
MMS/SiO2 spheres surface modification
Silanization of MMS/SiO2 spheres were performed by a procedure reported by Weihong Tan, etc. [6] briefly, MMS/SiO2 immersion in freshly prepared 1% (v/v) solution of distilled Trimethoxysilylpropyl diethylenetrimine (DETA) and 1 mM acetic acid for 1 h at 28 °C. The DETA modified spheres were thoroughly rinsed with deionized water to remove excess DETA. The silanized spheres were then treated with 10% succinic anhydride in dimethylformamide solution under argon atomesphere and stirred for 12 h.
Measuring methods
The structure and the properties of the MMS/SiO2 spheres were characterized by TEM, XPS, FTIR and VSM. TEM was performed on a JEM-2010 transmission Electron Microscope with an acceleration voltage of 200 kV. Samples for TEM were prepared by dispersing the MMS, MMS/SiO2 in ethanol and then applied drop-wise into copper grids. Dried MMS/SiO2 samples were submitted to ESCA 3600Shimadzu X-ray photoelectron spectroscope directly for surface analysis. For FTIR samples were mixed with KBr and pressed into tablets and the spectrum were obtained on a Bluker EQUINOX55 Fourier Transform Infrared spectrometer. Magnetization curve were measured at room temperature using a TM-VSM 2050HGC VSM. The stability of the MMS/SiO2 were evaluated by measuring the concentration of Fe2+ ion from samples dispersed in acidic or basic solution. The concentration of Fe2+ was recorded on Perkin-Elmer AA300 Atom Absorb Spectrophotometer to evaluate the stability of the powders in acidic or basic solution.
Results and discussion
Preparation of MMS/SiO2 spheres
Micron-size MMS/SiO2 spheres were prepared by addition of precursors to the mixture of MMS spheres, ammonia hydroxide and ethanol/water. The morphology and structure of the MMS and MMS/SiO2 were observed by Optical micrograph (OM) as shown in Fig. 1 and with TEM in Fig. 2. OM studies show that the both the MMS and MMS/SiO2 have a narrow size distribution. Transmission electron microscope studies confirmed the presence of a thin layer of SiO2 at the surface of the MMS spheres.
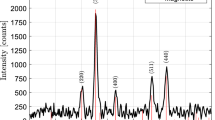
Figure 3 gives the broad and narrow scan XPS spectra of MMS/SiO2 spheres. The peak at 105 eV is the characteristic of Si 2P from SiO2. The peaks at 534.8 eV and 287.1 eV are assigned to O 1S and C1s, respectively. The date is consistent with our previously study [11]. There is no Fe 2P3/2 or Fe 2P1/2 peak in this graph. It can be explained that the Fe3O4 nanoparticls were imbedded inside of the MMS/SiO2 spheres. From this information, we can conclude that SiO2 is deposited on the surface of the MMS, forming a core/shell structure.
Figure 4 shows the FTIR spectra of MMS, MMS/SiO2 and MMS/SiO2–COOH. In the case of MMS/SiO2 (b), the broad band centered around 3440 cm−1 is assigned to the H–O–H stretching modes. The characteristic adsorption of the silica network is assigned as follows. The broad high-intensity band at 1,095 cm−1 is due to the asymmetric stretching bonds of Si–O–Si in Tetrahedron associated with oxygen motion of Si–O–Si antisymmetrical stretching. The band at 800 cm−1 is assigned to the Si–O–Si symmetric stretching, while the sharp band at 464 cm−1 corresponds to the Si–O–Si or O–Si–O bending mode. Compared with MMS curve (a), the main difference is that the MMS/SiO2 has a high intensive band at 1095 cm−1 and a 464 cm−1. Based on this, we conclude that SiO2 layer was covered on the surface of the MMS spheres. When compared with MMS/SiO2 (b), an intensive band at 1711 cm−1 was seen in the graph of MMS/SiO2–COOH (c), which is the characteristic of C=O, and thus the –COOH was functioned on the surface of the MMS/SiO2 spheres.
Magnetic properties of MMS/SiO2 spheres
The magnetic properties of the microspheres are of interest for all further applications. The magnetic properties include the saturation magnetization (Ms), the remanence (Mr) and coercivity (Hc). The magnetization measurements were performed using VSM at room temperature. There were two typical hysteresis loops shown in Fig. 5, which were measured in powdered state. Sample A is for MMS spheres and sample B for MMS/SiO2. From this graph we can see that the MMS and MMS/SiO2 are of superparamagnetism, and the saturation magnetization (Ms) of MMS and MMS/SiO2 are 1.6 and 1.35 emu/g, respectively. The difference of Ms can be ascribed to the deposition of SiO2 on the MMS. Assuming the difference of Ms is only due to the deposition of SiO2, the content of SiO2 is about 15.7%.
Stability tests of MMS/SiO2 spheres
The stability of the MMS/SiO2 spheres was studied by measuring its integrity under acid or base solutions. The protection of silica shell was studied by dispersing the MMS/SiO2 spheres in 0.01 M HCL or 0.01 M NaOH solution for 20 h. The solutions were then centrifuged and the supernatants were collected to measure the presence of Fe2+. The concentration of Fe2+ was measured by Perkin-Elmer AA300 Atom Absorb Spectrophotometer. In the experiment, Fe2+ was not found in the solution, indicating that Fe3O4 was well protected.
Conjugating MMS/SiO2 spheres to biomolecule
To investigate the feasibility of carboxyl groups on the surface of the MMS/SiO2 spheres, we performed the experiments of antibody adsorption.
To covalently attach antibody IgG to the MMS/SiO2 spheres, the carboxyl groups on the surface are activated with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide ester (NHS), before mixing with IgG at ambient temperature for 30 min. Solvent containing unlabeled antibody was decanted by collecting spheres with magnets and washing them for several times with phosphate buffer solution (PBS PH = 7.4). Fluorescence micrograph of the biomolecule decorated spheres is shown in Fig. 6, from which we can see that the MMS/SiO2 have ability linking to biomolecule.
Conclusions
MMS/SiO2 spheres were synthesized by a two-step process. First, the MMS were prepared by microemulsion polymerization of styrene, methacrylic acid and acryamide in the presence of magnetic fluid. Then silica shell was formed on MMS by addition of TEOS to the mixture of MMS, ammonium hydroxide and ethanol. The surface of the MMS/SiO2 was functioned with –COOH successfully. Characters of the MMS/SiO2 such as magnetism, morphology, structure and stability were studied. The saturation of magnetization decreased with reduced amount of MMS, but maintained superparamagnetism. The MMS/SiO2 spheres are stable in dilute acidic or basic solution. Bioconjugation ability was tested by connecting MMS/SiO2 to antibody IgG.
Reference
Hancock JP, Kemshead JT (1993) J Immunol Methods 164:51
Safarlk I, Safarikwa M, Forsythc S (1995) J J Appl Bacteriol 78:575
Lu QH, Yao KL, Xi D, Liu ZL, Luo XP, Ning Q (2006) J Magn Magn Mater 301:44
Khng HP, Cunliffe D, Davies S, Turner NA, Evgeny N-V (1998) Biotech Bioeng 60:419
Hilliard L, Zhao X, Tan WH (2002) Anal Chim Acta 470:51
Qhobosheane M, Santra S, Zhang P, Tan WH (2001) Analyst 126:1274
Tan WH, Wang KM, He XX et al (2004) Med Res Rev 24:621
Blum FD, Young EN, Smith G, Sitton OC (2006) Langmuir 22:4741
Beck JS, Varrali JC (1996) Curr Opin Solid State Mater Sci 1:76
Feng X, Fryxell GE, Wang LQ, Kim AY, Liu J (1997) Science 276:923
Du GH, Liu ZL, Xia X, Chu Q, Zhang SM (2006) J Sol-Gel Sci Techn DOI 10.1007/s10971-006-7780-5
Corrias A, Casula MF, Ennas G, Marras S, Navarra G, Mountjoy G (2003) J Phys Chem B 107:3030
Casas L, Roig A, Rodriguez E, Molins E, Teiada J, Sort J (2001) J Non-Cryst Sol 285:37
Grassel F, Labhsetwar N, Li D, Park DC, Saito N, Haneda H, Cador O, Roisnel T, Mornet S, Duguet E, Portier J, Etourmeau J (2002) Langmuir 18:8209
Graf C, Vossen DL-J, Imhof A, Blaaderen A-V (2003) Langmuir 19:6693
Vestal CR, Zhang ZJ (2003) Nano Lett 3:1739
Liu ZL, Yang XB, Yao KL, Du GH, Liu ZS (2006) J Magn Magn Mater 302:529
Liu ZL, Ding ZH, Yao KL (2003) J Magn Magn Mater 265:98
Liu ZL, Wang HB, Lu QH, Du GH, Peng L, Du YQ, Zhang SM, Yao KL (2004) J Magn Magn Mater 283:258
Acknowlegement
This work is supported by the National Nature Science Foundation of China under Grant nos. 10574047 and 10574048 and Doctor Foundation of China (No. 20030487003). This work was also supported by the National 973 Project under Grant. No. 2006CB921606.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Liu, P., Liu, C. et al. Characterization and application of magnetic P (St-co-MAA-co-AM)/SiO2 composite microspheres. J Mater Sci 42, 5147–5151 (2007). https://doi.org/10.1007/s10853-006-1284-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1284-0