Abstract
Te modified barium stannates BaSn1−xTexO3 (x = 0–15 mol%) provides an interesting result (i.e., ferroelectric phase transition) above room temperature. As we increase concentration of Te, dielectric anomalies (dielectric constant and loss) increases but Curie temperature (T c) shifted towards lower temperature side. Impedance analysis indicates the existence of both grain and grain boundary resistance nears the Curie temperature (∼225 °C–275 °C). At elevated temperature materials show a typically semiconducting nature (i.e., Negative temperature coefficient of resistance (NTCR)).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxides with perovskite structure having a general formula ABO3 (A = mono or divalent ions, B = tri, tetra, pentavalent ions) have extensively been studied in search of new materials for device applications. The physical properties of a perovskite can easily be tailored by making suitable substitution at A and/or B sites of the ABO3 structure. This has resulted into a large number of electrically neutral and charge deficient compounds [1–6]. An appropriate doping in BaSnO3 is expected to bring substantial modification in its lattice structure, defect energies, electronic disorder, redox behavior, thermal stability (even at high temperatures, i.e., T ≥ 1,000 °C), semi-conducting properties etc. making it suitable for a variety of applications such as sensors, thermally stable ceramic capacitors, computer memories, pyroelectric detectors, electrooptic modulator etc. Tireless efforts are still going on in search of new materials (pure/doped) having desirable properties such as high dielectric constant, low loss thermal stability etc. BaSnO3 is a perovskite structure material of special importance due to its low permittivity, semiconducting behavior and high thermal stability. The property of thermal stability up to 1,200 °C makes it quite attractive as a ceramic material for practical applications. But the low dielectric constant is a drawback imposing limitations for suitability of this material for same applications such as ceramic capacitors. This factor necessitated doping at A/B sites leading to tailoring of its dielectric properties. Some literature [7–12] reveals that substitution of Pb+2,Ti+4 etc. at this Ba/Sn sites results in substantial modification in its dielectric properties. It has been reported that Pb+2 doping/substitution at Ba-site causes the paraelectric to ferroelectric phase transition in BaSnO3 at −80 °C for doping concentration level higher than 30% [7]. This has provided a clear evidence of the possibility of improving dielectrical properties in BaSnO3 by appropriate doping. However, higher dielectric constant and low loss at ambient and higher temperatures are always a desired goal. It is well known that Te based peroveskite such as PbTeO3, SrTeO3, MgTeO3, BaTeO3 etc. show reversible phase transition at ambient and higher temperature [8–12]. The ferroelectric and high dielectric properties in these compound have been attributed to the presence of highly polarized Te+4 ion with unshared/paired electrons favoring the formation of a state of spontaneous polarization [12–18]. This aspect has motivated us to select Te as a dopant/substituent at the Sn site of BaSnO3 which may enhance dielectric properties and other physical properties. Further, since structural/microstructural changes in a system drastically modify their physical properties, a detailed study of electrical properties of Ba/Sn site substituted stannates assumes greater significance in order to have a complete picture of the electrical behavior of semiconducting stannate based ceramics. A survey shows that electrical properties of Ba/Sn site substituted BaSnO3 (with La at barium site and Co,Cr at the Sn Site) has been reported [18, 19].
The present paper reports the ferroelectric phase transition in Te-modified BaSnO3. In the present paper we are also analyzing the electrical properties of Te substituted BaSnO3 in different Sn/Te ratio over a range of concentration (x = 0–15 wt%) using a complex impedance spectroscopy (CIS) technique. The CIS approach of analyzing electrical properties gives an unambiguous result over a range of temperature and frequencies. The results are basically independent of sample geometrical factors and enable us to establish a correlation of the electrical properties with the sample microstructure.
Experimental
Appropriate stoichiometric of the precursors (BaCO3, TeO2, and SnO2) of high purity were weighed and initially mixed mechanically in an agate mortar for 2 h. Subsequently, it was calcined in alumina crucible at 1,000 °C for 12 h. The calcined powder was thoroughly mixed again and reclined at 1,150 °C for 12 h. The calcined powder so obtained was cold pressed into cylindrical pellets of diameter 10 mm and thickness1–2 mm with polyvinyl alcohol (PVA) as the binder, using a hydraulic press at a pressure of ∼3 × 107 kgm−2. The pellets were then sintered in an air atmosphere at 1,200 °C for 12 h, and then polished with fine emery paper to make their faces flat and parallel. The pellets were finally coated with conductive silver paint and dried at 150 °C for 2 h before carrying out impedance measurements.
X-ray diffraction (XRD) data of the materials were obtained in the wide range of Bragg angles (2θ) (20–80°) at a scan speed of 2° min−1 by an X-ray diffractometer (Miniflux, Rikagu, Japan) using CuKα radiation (λ = 1.5418 Å) at room temperature. The impedance studies were carried out at an input signal level of 1.3 V in the temperature range of 35–500 °C using a computer-controlled impedance analyzer (HIOKI LCR Hi TESTER, Model: 3532) in the frequency range of 100 Hz to 1 MHz.
Results and discussion
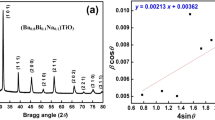
X-ray diffraction (XRD) pattern of Te-modified BaSnO3 is shown in Fig. 1. Sharp single peaks, which are almost similar to that of BaSnO3 with a change in peak intensity, confirm the formation of single-phase polycrystalline compounds. A shift in XRD peak positions compared to that of BaSnO3 on Te-doping confirms substitution at the Sn site. All the diffraction peaks of the material have been indexed and lattice parameter (a for cubic) of the samples was calculated using a computer program package POWD [20]. The least-squares refined lattice parameter and volume for different concentration of Te (in BaSnO3) are given in Table 1. These are in good agreement with the earlier reports [6, 7, 20]. All the compounds have the cubic unit cell at room temperature, which shows that the Te+4 ions (having almost similar size to that of Sn+4 ions) can easily be substituted at the Sn+4 site. Figure 2 shows the temperature dependence of dielectric properties (permittivity and loss tangent) for both the pure and tellurium doped BaSnO3 at 10 kHz. The relative permittivity of pure BaSnO3 has been observed to be very low even at higher temperatures at 10 kHz frequencies. The nature of variation of permittivity as a function of temperature indicates appearance of a peak at a particular temperature at 10 kHz for different doping concentration of Te in BaSnO3. Substantial modification in the value of permittivity has been observed on doping with Te and the changes occurring in the pattern when compared with that of the undoped BaSnO3 are: (i) drastic increase in the permittivity with a peak at a particular temperature, (ii) increase in the height of the peak (iii) a shift in the peak position towards the lower temperature side and (iv) increase in the sharpness of the peak (marked reduction in the broadening of the dielectric peak) on increasing the doping concentration of Te in BaSnO3 varying in the range of x = 5–15%. The appearance of the peak in the pattern of permittivity variation with temperature (Fig. 2) on Te doping in BaSnO3 can be attributed to the ferroelectric–paraelectric phase transition.
An enhancement in the magnitude of permittivity by ∼25 times at 10 kHz on Te doping may be attributed to the presence of highly polarized Te+4 ion having unshared/paired electrons favoring the formation of a state of spontaneous polarization in the doped material. This observation is in good agreement with the pervious reports on the presence of phase transition corresponding to the ferroelectric–paraelectric in telluride compounds with perovskite (ABO3) structure [8–10]. Figure 2 shows the variation of tangent loss with temperature at 10 kHz. At low frequency (1 kHz) all the Te-modified compounds exhibits low loss. But at higher temperature the dielectric loss increases rapidly with temperature tending to attain a saturation value at elevated temperatures.
Figure 3 represents the complex impedance spectra of the Te modified BaSnO3 for the different Te concentration. It shows the evidence of two semi-circular arcs in the Curie temperature range (near T c) for all the concentration. It has been attributed to the presence of grain boundary. The assignment of the two semi-circular arcs to the electrical response due to bulk resistance and grain boundary is consistence with the “brick-layer model” for a polycrystalline material [21, 22]. The curves (Fig. 4) show the broad and asymmetric peaks, at around the frequency of maximum just below and above the Curie temperature. Maximum and inflection points are observed. Both characteristics are a function of temperature and frequency. In the investigated temperature range two inflections are identified. These inflections are vanishes at the higher temperature region. The first inflection is shown at low frequency (≤1 kHz), while the second one is observed at around (≥10 kHz) below 300 °C. However, at temperature ≥300 °C, only a single unique peak is observed at high frequency region (≥10 kHz). Blow 300 °C, the first impedance loss peak at low frequency is correlated to the grain boundary contribution, while the second one is correlated with the bulk response. In addition the curve shapes suggest that there are two type of relaxation with most frequent value at very similar frequency. The grain boundary effect vanishes at the higher temperature and single peak observed due the bulk resistance of the material. Moreover, the relaxation frequency is shifted towards higher frequency side with increasing temperature. The phenomenon is well matched with the microstructure and Complex impedance (Nyquist) plot of the related materials. Figure 5 shows the comparison of hysterisis loop of all the compounds at room temperature. As the samples could not be sufficiently poled because of the dielectric break down, we did not get the proper loops. However, area of loops was found decreasing with rise in temperature. Since the transition temperature of the present investigating material is greater than 250 °C that’s why we are unable to get the behavior of hysteresis area exact at the transition temperature. BaSnO3 does not show any phase transition above room temperature, and hence there is no hysteresis loop. For Te modified BaSnO3 we have hysteresis loop suggesting the phase transition and ferroelectric properties in the compounds at room temperature.
Conclusion
A preliminary structural analysis indicates that the cubic crystal structure of BaSnO3 remains unaffected even on Te-doping with a selected range of concentration (up to 15 mol%). The permittivity data for a polycrystalline material BaSn1−xTexO3 (x = 0–15 mol%) show a sharp ferroelectric–paraelectric phase transition at around 535–555 K for different Te concentration. The Curie temperature is shifting towards low temperature side. In the Curie temperature region material shows the presence of grain and grain boundary contribution in the electrical conductivity of the material. An impedance spectrum reveals the two-inflection point at different frequency region that confirmed the grain boundary effect (Fig. 4). The decrease in bulk resistance with increasing temperature shows a typical semiconducting behavior of the material (i.e., Negative temperature coefficient of resistance, NTCR). Hysterisis loop indicates ferroelectrics phase transition in Te modified BaSnO3.
References
Claessen R, Smith MG, Goodenough JB (1993) Phys Rev B 47(4):1788
Lu W, Jiang S, Zhou D, Gong S (2000) Sensor Actuator A: Phys 80(1):35
Cava RJ, Gammel P, Batlog B, Krajerki JJ, Peck WF Jr., Rupp LW Jr., Felder R, Van Dover RB (1990) Phys Rev B 42(7):4815
Elermon Y (1993) Turkish J Phys 17(7):465
Parida SC, Banerjee A, Das S, Prasad R, Singh Z, Venugopal V (2002) J Chem Thermody 34:527
Udawatte CP, Kakihana M, Yoshimura M (2000) Solid State Ionics 128:217
Raevskii IP, Prokopalo OI, Kolesnikove SG (1983) Rostov-on-Don 53:1175
Jayaraman V, Mangamma G, Gnasekaram T, Periaswami G (1996) Solid Solid State Ionics 86:1111
Rai RS, Sharama S, Choudhary RNP (2002) Ferroelectric 275:11
Sharma S, Choudhary RNP (1999) J Electroceram 4:443
Rai R, Sharama S, Choudhary RNP (2002) J Mater Sci Lett 21:297
Singh NK, Choudhary RNP (2000) Ferroelectrics 242(1–4):89
Young LM (1979) J Mater Sci 14:1579
Yamada T, Iwasaki H (1979) J Appl Phys (44):1579
Tu CS, Siny IG, Schmidt VH (1994) Phys Rev B 49:11550
Lee KS (1996) J Phys Chem Solids 57:333
Ortiz E, Vargas RA, Mellander BE (1998) J Phys Chem Solids 59:305
Shimizu F, Takashige M, Sawada S, Yamaguchi T (1993) J Phys Soc Jpn 62:2964
Luiz JM, Matos JR, Giolito I, Ionashiro M (1995) Thermochimica Acta 254:209
POUWMOLT An Interactive Powder Diffraction Data Interpretation and Indexing ProgramVersion 2.1, E.Wu, School of Physical Sciences, Flinder University of South Australia, Bradford Park, SA 5042, Australia
Goto Y (1980) J Phys Soc Jpn 50:538
Shockley W, Read WT (1952) Phys Rev 87:239
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Choudhary, R.N.P. & Singh, B.P. Structural, dielectric and electrical properties of Te modified barium stannates using impedance analysis. J Mater Sci 42, 8306–8310 (2007). https://doi.org/10.1007/s10853-006-1244-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1244-8