Abstract
The hydrothermal reactions of fibrous H2Ti4O9 particles with Ba(OH)2 solution in the presence of cationic surfactants of n-hexadecyltrimethylammonium hydroxide (HTMA-OH) and n-hexadecyltrimethylammonium bromide (HTMA-Br) were investigated in a temperature range of 150–250 °C. H2Ti4O9 phase with layered structure was transformed to BaTiO3 phase in the Ba(OH)2–(HTMA-OH) and the Ba(OH)2–(HTMA-Br) solutions, and partially transformed to anatase phase in the Ba2+-free HTMA-OH and HTMA-Br solutions by topotactic structural transformation reactions under the hydrothermal conditions. The cocoon-like BaTiO3 and titanium oxide particles were obtained after the hydrothermal reactions in the Ba(OH)2–(HTMA-OH) and HTMA-OH solution, respectively. These cocoon-like particles were formed by assembling the fibrous particles in the surfactant solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Barium titanate (BaTiO3) is a well known electroceramic material widely utilized in the manufacture of multilayer capacitors, thermistors, and electro-optic devices due to its high dielectric permittivity and ferroelectric properties [1, 2]. BaTiO3 particles have been prepared by using various methods. High purity BaTiO3 with controlled particle size can be prepared by using the hydrothermal [3–5] and sol–gel methods [6–8]. However, these methods usually give the particles with a spherical or cubic shape, meaning it is difficult to prepare BaTiO3 particles with special shape, such as fibrous and plate-like particles which have potential application in the preparation oriented dielectric ceramics.
Soft chemical synthesis using host-guest reactions is a unique and useful method for inorganic material synthesis. In the soft chemical synthesis, a compound with layered structure or open structure can be used as the precursor. The layered structure of the precursor can be transformed to a desired structure by an in situ topotactic structural transformation reaction, and the morphology of the precursor can be retained after the reaction [9–12]. This means that the morphology of the product is dependent on that of the precursor, which is different from normal methods, e.g. sol–gel method and hydrothermal method, where the crystal particle morphology is almost independent of the morphology of the precursor. Hydrothermal reaction is useful for the structural transformation in the soft chemical synthesis. Fibrous BaTiO3 and PbTiO3 particles have been prepared by hydrothermally reacting a fibrous layered hydrous potassium titanate (2K2O·11TiO2·3H2O) in Ba(OH)2 and Pb2O(OH)2 solutions, respectively [13–15]. These fibrous BaTiO3 and PbTiO3 particles show high degree crystal-axis orientation and anisotropic dielectric properties. Our previous study has indicated that plate-like and fibrous anatase and BaTiO3 particles can be prepared by hydrothermally reacting H+-form layered titanates of H1.07Ti1.73O4 and H2Ti4O9 with plate-like and fibrous particle morphologys in distilled water and Ba(OH)2 solution, respectively [16, 17]. The transformation reactions from the layered phase to the anatase and BaTiO3 phases are in situ topotactic reactions. The anatase and BaTiO3 particles prepared by this method also show high degree crystal-axis orientation properties.
In the present paper, we describe preparation of BaTiO3 and titanium oxide from fibrous H2Ti4O9 precursor with a layered structure by hydrothermal reaction in the presence of a cationic surfactant. In this reaction system, the fibrous product particles can be assembled, and shaped into cocoon-like particles, which provides a new process for modification of particle morphology in the soft chemical synthesis.
Experiment
Fibrous layered K2Ti4O9 (Otsuka Chemical Co., Ltd) with dimensions of 0.3–0.6 μm in diameter and 10–20 μm in length was used as the precursor. The fibrous K2Ti4O9 particles (10 g) were treated in a 1 M HNO3 solution (1 L) for 1 day at room temperature to exchange K+ in the layered structure with H+. The acid-treatment was repeated for twice to complete the ion-exchange reaction, and fibrous H2Ti4O9 particles were obtained after the ion-exchange reaction. The ion-exchanged sample was washed with distilled water and dried at room temperature.
The fibrous H2Ti4O9 (0.147 g) was added into a 0.1 M cationic surfactant solution (15 mL) of n-hexadecyltrimethylammonium hydroxide (HTMA-OH) or n-hexadecyltrimethylammonium bromide (HTMA-Br), and stirred at room temperature for 2 h, and then a desired amount of Ba(OH)2·8H2O solid was added into the solution to adjust the concentration of Ba(OH)2 to 0, 0.1, 0.2, and 0.3 M, respectively. Thus, the Ba/Ti molar ratios in the reaction system are controlled to be 0, 1, 2, and 3, respectively. The mixture (15 mL) was placed in a Teflon-lined, sealed stainless steel vessel (30 mL of inner volume), and then hydrothermally treated in a temperature range of 150–250 °C for 24 h under autogenously pressure and stirring conditions. The product was filtered, washed with hot distilled water, and then dried at 80 °C for 24 h.
The crystal structures of samples were investigated by using a powder X-ray diffractometer (Rigaku Rotaflex RAD-RC). The particle size and morphology were characterized by scanning electron microscopy (SEM, Hitachi S-530).
Results and discussion
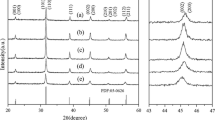
K2Ti4O9 has a layered structure with K+ and water molecules in the interlayer space, and the K+ are ion-exchangeable [9]. After the acid-treatment, K+ were exchanged with H+, but retained the layered structure (Fig. 1a) with a basal spacing of 0.791 nm, and retained its fibrous particle morphology (Fig. 2a). When H2Ti4O9 was treated in a 0.1 M Ba(OH)2 solution at room temperature, the basal spacing of the layered titanate increased from 0.791 to about 1.1 nm (Fig. 1b), indicating that Ba2+ can be intercalated into the interlayer space by a Ba2+/H+ ion-exchange reaction. An impurity phase of BaCO3 was also observed in the sample, due to the reaction of Ba(OH)2 solution and CO2 gas in air. When H2Ti4O9 was treated in HTMA-OH solution at room temperature, the basal spacing of the layered titanate increased from 0.791 to 1.13 nm (Fig. 1c), indicating that HTMA+ can be intercalated into the interlayer space by a HTMA+/H+ ion-exchange reaction. The HTMA+ in the interlayer space can be exchanged with Ba2+. After treatment of the HTMA+-exchanged layered titanate in Ba(OH)2 solution, product shows a same XRD pattern as that of Ba2+-exchanged titanate (Fig. 1d). The above results reveal that Ba2+ can be intercalated easily into the interlayer space of the layered titanate by the ion-exchange reaction, which is important for the soft chemical synthesis of BaTiO3.
X-Ray diffraction patterns of (a) H2Ti4O9 used as the precursor, samples obtained by reacting H2Ti4O9 in (b) 0.3 M Ba(OH)2 solution and (c) 0.1 M HTMA-OH solution, respectively, and (d) sample after reacting sample (c) in 0.3 M Ba(OH)2 solution, for 1 day at room temperature. ■: H2Ti4O9 phase; ▲: Ba2+-exchanged layered phase; ●: HTMA+-exchanged layered phase; ▽: BaCO3 phase
SEM photographs of (a) H2Ti4O9 used as the precursor and (b)–(h) products obtained by hydrothermally reacting H2Ti4O9 under stirring conditions for 1 day. (b), (c) and (d) in 0.1 M HTMA-OH solution at 150, 200 and 250 °C, respectively; (e) and (f) in 0.3 M Ba(OH)2–(HTMA-OH) solution at 150 and 250 °C, respectively; (g) and (h) in 0.1 M HTMA-Br solution and 0.3 M Ba(OH)2–(HTMA-Br) solution, respectively, at 150 °C
Figure 3 shows XRD patterns of samples obtained by hydrothermally reacting H2Ti4O9 in Ba(OH)2–(HTMA-OH) solutions at 150 °C under stirring conditions. Most of the layered titanate phase was transformed to a BaTiO3 phase, and only very small amount of unreacted layered titanate phase remained after the reaction in the Ba(OH)2–(HTMA-OH) solution. The amount of unreacted layered titanate phase decreased with increasing the concentration of Ba(OH)2. The layered titanate phase partially transformed to anatase phase in Ba2+-free HTMA-OH solution (Fig. 3a). The formation of BaTiO3 phase increased with increasing the reaction temperature in the Ba(OH)2–(HTMA-OH) solution. The single BaTiO3 phase was obtained in 0.2 and 0.3 M Ba(OH)2–(HTMA-OH) solutions at 200 °C. At 250 °C the single BaTiO3 phase was obtained in 0.1, 0.2, 0.3 M Ba(OH)2–(HTMA-OH) solutions, but most of the layered titanate phase remained even after reaction at 250 °C in the Ba2+-free HTMA-OH solution (Fig. 4).
The above results indicate that Ba(OH)2–(HTMA-OH) solution shows an almost same reactivity for the formation of BaTiO3 phase as that of Ba(OH)2 solution, while HTMA-OH solution shows a lower reactivity for the formation of the anatase phase than that of distilled water under the hydrothermal conditions [17]. The BaTiO3 phase is formed mainly by an in situ topotactic transformation reaction similar to the case in the H2Ti4O9–Ba(OH)2 reaction system, in which Ba2+ migrate into the crystal bulk of layered titanate through the interlayer space, and then react with titanate layers to form BaTiO3 in the crystal bulk. The transformation from H2Ti4O9 phase to anatase phase is a dehydration reaction. The H2Ti4O9 phase can be transformed completely to anatase phase after reaction in distilled water at 200 °C. The XRD results suggest that the H2Ti4O9 phase is easier to be dehydrated into anatase phase in the neutral solution than that in the alkaline solution under the hydrothermal conditions. The HTMA+ in the interlayer space can stabilize also the layered structure.
The hydrothermally reacted products show an interesting morphology. The fibrous particles aggregated together to form cocoon-like particles with smooth surface after the hydrothermal reaction in the Ba2+-free HTMA-OH solution at 150 and 200 °C (Fig. 2b and c). Cocoon-like BaTiO3 particles can be obtained by the hydrothermal reaction in the Ba(OH)2–(HTMA-OH) solutions at 150 and 200 °C (Fig. 2e). At 250 °C, however, no cocoon-like particle was observed in Ba2+-free HTMA-OH or Ba(OH)2–(HTMA-OH) solutions. At 250 °C when H2Ti4O9 was hydrothermally reacted in Ba2+-free HTMA-OH solution, the particles with similar morphology to the precursor were obtained, where without aggregation occurred (Fig. 2d). In the Ba(OH)2–(HTMA-OH) solutions, the fibrous morphology of the precursor was broken down to smaller particles (Fig. 2f), due to a dissolution-deposition reaction on the surface of the particles, similar to the normal hydrothermal reaction [16]. The dissolution-deposition reaction changes the fibrous morphology of the precursor.
When H2Ti4O9 was hydrothermally reacted in Ba(OH)2–(HTMA-Br) solutions, BaTiO3 phase was formed also, similar to the case of that in Ba(OH)2–(HTMA-OH) solutions (Fig. 5). In the Ba2+-free HTMA-Br solution, the anatase phase is formed more easily than that in the Ba2+-free HTMA-OH solution. A layered titanate phase with a large basal spacing of 2.57 nm was observed (Fig. 5a), suggesting more HTMA+ were intercalated into the layered titanate under the hydrothermal conditions than that at room temperature, which caused the increase of the basal spacing of the layered phase. The fibrous particles assembled together also in the Ba(OH)2–(HTMA-Br) solutions, but the cocoon-like particle was not observed (Fig. 2g and h).
X-ray diffraction patterns of products obtained by hydrothermally reacting H2Ti4O9 in (a) 0, (b) 0.1, (c) 0.2, and (d) 0.3 M Ba(OH)2–(HTMA-Br) solutions, respectively, at 150 °C for 1 day under stirring conditions. □: layered titanate phase with a basal spacing of 2.57 nm; △: anatase phase; ○: BaTiO3 phase
The above results indicate that HTMA-OH and stirring are necessary to form the cocoon-like particles. In the particle assembling process, HTMA+ plays an important role in the formation of the cocoon-like particles. The surfactant ions can be adsorbed on the surface of the H2Ti4O9 fibers, and the interaction of the hydrophobic group promotes the assembling of the fibrous particles. Under the stirring conditions, the assembled fibrous particles are shaped into the cocoon-like particles. In the Ba(OH)2 solution, the layered phase of the cocoon-like particles is transformed to BaTiO3 phase by the in situ topotactic reaction, but the cocoon-like particle morphology is retained after the reaction.
Conclusions
The results of this study indicate that the hydrothermal soft chemical process is useful for the preparation of BaTiO3 and titanium oxide with special morphology. The cocoon-like BaTiO3 and titanium oxide particles can be prepared by hydrothermally reacting fibrous H2Ti4O9 particles in Ba(OH)2–(HTMA-OH) and HTMA-OH solutions, respectively, in the temperature of 150–200 °C. The cocoon-like BaTiO3 and titanium oxide particles are formed by assembling fibrous BaTiO3 particles and fibrous titanium oxide particles, respectively. The HTMA+ in the reaction system plays an important role in the particle assembling process. The transformation reaction from the H2Ti4O9 phase to the BaTiO3 phase is topotactic structural transformation reaction.
References
Phule PP, Rispud SH (1990) J Mater Sci 25:1169
Burfoot JC, Taylor GW (1979) Polar dielectrics and their applications. Macmillan, London
Kutty RN, Vivekanandan R, Murugaraj P (1998) Mater Chem Phys 19:533
Oledzka M, Brese NE, Riman RE (1999) Chem Mater 11:1931
Wu M, Long J, Wang G, Huang A, Luo Y, Feng S, Xu R (1999) J Am Ceram Soc 82:3254
Hernandez BA, Chang K, Fisher ER, Dorhout PK (2002) Chem Mater 14:480
Shimooka H, Kohiki S, Kobayashi T, Kuwabara M (2000) J Mater Chem 10:1511
J-F Campion, DA Payne, HK Chae And Z Xu, Ceram Trans, 22 (1191) 477 Ceramic Powder Science Iv Edited By Hirano, S, Messing, GL And Hausner, H, American Ceramic Society, Westerville OH USA
Sasaki T, Watanabe M, Fujiki Y, Kitami Y (1994) Chem Mater 6:1749
Rouxel J, Tournoux M, Brec R (1994) Soft chemical routes to new materials. Trans Tech Publications, Aedermansdorf
Stein A, Keller SW, Mallouk TE (1993) Science 259:1558
Feng Q, Kanoh H, Ooi K (1999) J Mater Chem 9:319
Ohara Y, Koumoto K, Yanagida H (1985) J Am Ceram Soc 68:C-108
Ohara Y, Koumoto K, Yanagida H (1994) J Am Ceram Soc 77:2327
Ohara Y, Koumoto K, Shimizu T, Yanagida H (1994) J Ceram Soc Jpn 102:88
Feng Q, Hirasawa M, Yanagisawa K (2001) Chem Mater 13:290
Feng Q, Yanagisawa K, Yamasaki N (2001) High Pressure Res 20:149
Acknowledgement
This work was supported in part by Grants-in-Aid for Scientific Research (C) (No.13650894) from Japan Society for the Promotion of Science and the Murata Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, Q., Hirasawa, M., Kajiyoshi, K. et al. Hydrothermal soft chemical synthesis of BaTiO3 and titanium oxide with cocoon-like particle morphology. J Mater Sci 42, 640–645 (2007). https://doi.org/10.1007/s10853-006-1142-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1142-0