Abstract
The relationship between dislocations and mechanical properties of single hemp fibres (Cannabis sativa L. var. Felina) was studied using a microtensile testing setup in a 2-fold approach. In a first investigation the percentage of dislocations was quantified using polarized light microscopy (PLM) prior to microtensile testing of the fibres. In a second approach PLM was used to monitor the dislocations while straining single fibres. The first part of the study comprised 53 hemp fibres with up to 20% of their cell wall consisting of dislocations. For this data set the percentage of dislocations did not affect the mechanical properties. In the second part of the study it was found that dislocations disappeared during tensile testing, and that they did not reappear until several weeks after failure. A strain stiffening effect due to the straightening of the dislocations was not observed. It is possible that the former positions of the dislocations functioned as locations for crack initiation. However, the crack does not propagate transversely all the way trough the dislocation but results in a shear failure between the microfibrils. In rheological studies fibres were strained at constant stress levels, and dislocations that had disappeared did not reappear during that period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
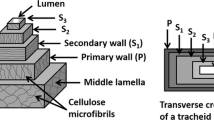
Natural fibres such as wood, flax and hemp contain dislocations, i.e. regions in the fibre cell wall where the angle of the microfibrils relative to the fibre axis differs from the angle found in the surrounding cell wall. Dislocations are also known as nodes, kinks, kink bands or slip planes, but here we use the word dislocations in accordance with Nyholm et al. [1]. Dislocations have been found in never dried fibres carefully extracted from hemp [2]. However, others have reported such carefully extracted fibres to be virtually free of dislocations [3]. Dislocations can occur in various forms. Some protrude over neighbouring fibres, and result in kinks, suggesting that some event either before or after harvest caused a common damage. Other dislocations affect only part of the microfibrils within a single fibre. Dislocations appear to be located only in the secondary cell wall, not in the primary wall [4]. Groups of up to 15 dislocations have been found in flax [5]. In such a group a number of dislocations are situated close to each other along a fibre.
The reason for the occurrence of dislocations already in the living plant is unknown, but damages originating from swaying of the plant in the wind or growth stresses have been suggested as possible causes [6, 7]. According to Hughes et al. [2], calculations for unidirectional composites indicate that an average misalignment of 3° or more suffices for dislocations to evolve in compression due to “local buckling instability associated with plastic deformation of the matrix”. From this they speculate that a microfibril angle of 2° might be enough to make flax and hemp susceptible to this mechanism. With regard to the effects of post-harvest treatment (drying and various steps in defibration), there is no doubt that dislocations are normally formed and/or enhanced during the processing. It has long been known that compressive stress applied in the longitudinal direction of the fibre induces dislocations [8]. For flax, it has been found that compressive stress results in dislocations in the secondary fibre wall, causing the primary cell wall to buckle out [4].
Few studies have addressed the effect of dislocations on the mechanical properties of individual natural fibres, and furthermore these studies are based on no more than tens of fibres. Based on a study of wood pulp single fibres, Page et al. [9] claimed that “wrinkles and nodes... initiate failure and lower the fibre strength”, but they showed no quantitative data that support this conclusion. Baley [5] investigated the relationship between tensile stress and the number of dislocations per mm fibre length for 48 flax fibres. The test span used was 1 mm. No interrelation was found. Bos et al. [3] determined the tensile strength of 25 elementary flax fibres isolated by hand and compared to the strength of 25 standard decorticated fibres (span length 3 mm). They found mean ultimate tensile stress values of 1834 ± 900 MPa and 1522 ± 400 MPa, respectively. The number of dislocations per mm fibre length or their severity was not determined, but the hand isolated fibres were reportedly virtually free of kink bands, in contrast to what was the case for the standard decorticated fibres. However, as the two types of fibres were not solely different with regard to the frequency of dislocations, the difference in strength could be due to other differences between them. Davies and Bruce [10] studied the relationship between tensile properties and the amount of dislocations for flax and nettle fibres (74 and 50 fibres, respectively). They quantified the amount of dislocations as the so-called “damage”, which they defined as the total area of the dislocations as a fraction of the total fibre area. A large scatter was found in the results, but a weak negative relationship seemed to be present for both species. In terms of flax the range of damage values ranged from 0 to approximately 0.65. For “damage” values below 0.2 their results showed a large variation in strength for the same “damage” value, a scatter that appeared to be a little smaller for higher damage values. However, this may alternatively be due to a lack of data for these values (only eight values were above 0.2). Their explanation to their results was that there is a lower strength limit which is independent of the “damage” level, while the upper strength limit decreases with increased damage, i.e. that also nearly undamaged fibres may have a weak point, while very strong fibres are not found between heavily damaged fibres. The problem of the “damage” parameter is thus, still according to Davies and Bruce [10], that it expresses the extent rather than the severity of the damaged areas.
In the study reported here we investigated the relationship between dislocations and the mechanical properties of individual hemp fibres. Since cellulose is birefringent, dislocations can be visualized using polarized light microscopy (PLM). If a fibre is viewed under crossed polarisers and oriented so that the longitudinal direction of the fibre is parallel to the vibrational direction of one of the two filters, the bulk fibre wall will appear dark, while dislocations will light up. By monitoring a fibre by PLM during tensile testing using a specially designed and adapted tensile testing stage, it was possible to study the behaviour of the dislocations during straining of the fibre.
Material and methods
Hemp fibres
The hemp (Cannabis sativa L., var. Felina) fibres were isolated from stems of hemp plants grown in Denmark during the 2001 growth season using 32 kg seeds per ha. The plants were dried after harvest, but not retted, and stored at room temperature. The hemp stems were provided by the Danish Agricultural Research Center (DJF).
Prior to the isolation, a piece of hemp stem was soaked in water, and individual fibres were carefully hand isolated under a stereo microscope (25 × magnification) using precision tweezers. The individual hemp fibres used here were 30–50 μm in diameter and 10–20 mm long.
Microtensile testing after determining the percentage of dislocations
Tensile tests were carried out using a microtensile testing device combined with video extensometry. The single fibres were glued onto a sufficient stiff foliar frame using cyanoacrylate glue. The free length of the fibres was determined using light microscopy and the average length was ∼1 mm. Thereafter the percentage of dislocations was determined for the free length of the fibre by means of total area of dislocations relative to the area of the free part of the fibre as seen in the polarized light microscope when the longitudinal axis of the fibre was parallel to the vibrational direction of one of the two filters. The theoretical minimum value of this parameter would be 0 for a fibre completely free of dislocations, and the theoretical maximum value would be 1 for a fibre with nothing but dislocations in the cell wall. As mentioned above, Davies and Bruce [10] used the term “damage” for a parameter similar to the one used here. Subsequently the foliar frame carrying the single fibre was fixed onto the moveable table of a microtensile testing device by a pinhole assembly [11]. A load cell with a maximum capacity of 1500 mN was used and a test speed of 2 μm/s was chosen. Elongation was recorded by video-extensometry for a sufficient strain measurement. The fibres were tested at 20 °C and 65% relative humidity. For stress calculations the fibres were cut with razor blades close to the fractured surfaces and subsequently cross sections were determined in an Environmental Scanning Electron Microscope in the Low Vacuum mode. 53 fibres were successfully measured.
In-situ microtensile testing combined with polarized light microscopy
Tensile experiments under polarized light were performed using a microtensile testing device specially designed for this purpose. The single fibres were glued onto a foliar frame as described above, the free length of the fibres was ∼3 mm. The foliar frames with the single fibres were glued onto metal adaptors mounted on the load cell and on the motor driven side of the microtensile testing device. A load cell with a maximum capacity of 50 N was used and a low test speed of 0.5 μm/s was chosen in order to investigate the changes with sufficient accuracy. Each 10 or 20 mN the test speed was set to zero to allow PLM images to be taken. The microscope used was a Leica DMRXA2 equipped with a 20× objective. The digital camera was a Leica DFC480, and images with size 2560 × 1920 pixels were taken. Successful results were obtained for six fibres. For three additional fibres the rheological properties were measured by increasing force until a certain level, and then keeping it for 20 min while monitoring dislocations using PLM. All experiments were carried out under laboratory conditions. Strain was determined from the images taken during the tensile test. Three reference points on a fibre were chosen, and their positions marked on all images of the fibre. From these positions the average strain could be estimated for each force level. PLM images were taken of fibres before, during and after the tensile testing. For some fibres images were also taken approximately two weeks and approximately two month after they were strained to failure.
Results and discussion
The relationship between the amount of dislocations and the mechanical properties, i.e. the ultimate stress and the modulus of elasticity (MOE) are shown for 53 hemp fibres in Fig. 1. The figure shows a large scatter in results, and no interrelation between the relative fraction of dislocation area and the two different mechanical properties are seen. However, the largest relative dislocation area encountered in the data set is just over 0.2. It is possible that an effect on the mechanical properties would have been seen if more severely damaged fibres had been included [10]. Even though no Gaussian distribution exists for the sample, a mean and a standard deviation are given in order to allow comparison with data from the literature. The mean ultimate stress of the hemp fibres was calculated to 1735 ± 723 MPa, whereas the MOE was 24.9 ± 10.6 GPa. The ultimate stress is in good agreement with the data given by Bos et al. [3] determined for flax fibres isolated by hand (1834 ± 900 MPa). Robson and Hague [12] reported a tensile strength of ∼900 MPa and a MOE of ∼25 GPa for hemp fibres. Our data show a higher ultimate stress but the MOE is in accordance with their results. Remarkably, the scatter of mechanical properties of hemp and flax is much larger than in wood fibres [13, 14].
Figure 2 shows PLM images of a fibre during straining as well as the corresponding force/strain curve. The figure shows that dislocations disappear gradually during straining, and that they disappear long before failure; in the given example they no longer light up at around 50% of the final stress. Even very large dislocations are removed by the straining. However, the respective force-strain curve indicates no tensile stiffening. Thus, the straining of dislocations might have no effect on the stiffness of the fibre or, alternatively and more likely, the effect is not detectable due to a small impact and superimposition of events. From the initial stage up to half of the ultimate stress dislocations disappear gradually. Since <20% of the fibre is affected, an influence on the fibre stiffness is given, but does not lead to an apparent softening or stiffening. For stresses above the level at which the dislocations disappear, the former positions of them may in most cases still be discerned as dark lines. Rotation of fibres in the plane during PLM did not reveal a position where the straightened dislocations could be made to light up differently than the bulk cell wall. This might indicate that the affected microfibrils are almost aligned but still partly distorted. Thus, even beyond the stage of visible dislocations they might still be marginally affecting the tensile behaviour. Equally to a gradual decrease of this “softening” effect, fibres go through an increasing rate of deformation. In conjunction, this might result in an almost straight force-strain curve until failure.
The dark remaining marks indicate regions where the locally rearranged microfibrils still contain transverse distortions and/or a disturbed pattern of inter-fibrillar bonding. If this assumption is correct, the former positions of the dislocations might still function as points for crack initiation. A typical fractured surface of a hemp fibre after tensile failure is seen in Fig. 3a. The image shows that the fibre failed in shear, where different layers within the cell wall are separated from each other; fibrillation is also seen. This type of failure agrees with the layered cell wall structure identified in the S2 cell wall of hemp [15], and with a small relative volume fraction of lignin in the cell wall [12]. In Fig. 3b a shear plane within the cell wall is shown. An “inner” core can be identified which is partly separated from the outer fibrillar structure. Two distinct distortions cross the inner core transversely. Presumably, these two lines mark a former dislocation indicating a transverse distortion and a disturbed pattern of inter-fibrilar bonding after straining. For flax it has earlier been found that the inter-fibrillar bonding is the weakest link within a fibre wall, and that the inter-fibrillar bonds are the first to break when compressive stress is applied [4]. In terms of tensile stresses this effect should be less pronounced and a fibre with a dislocation protruding over the entire cross section will probably not break in a brittle fracture along the dislocation. But the former position of the dislocation may be a point at which a crack is initiated and gains access to weaker inter-fibrillar bonds within the S2 layer, resulting in a shear failure and fibrillation. However, as already discussed we could not measure a distinct change in MOE due to the disappearance of dislocations. This means that surprisingly, the stiffness of the hemp fibres remains the same or changes only marginally, no matter whether the dislocations are strained to alignment with the surrounding wall or not.
Figure 4 shows PLM images of a fibre before tensile testing, immediately after tensile testing, approximately 2 weeks after, and approximately 2 months after tensile testing. For a long time it seemed that the dislocations do not reappear after failure, but surprisingly after 2 months they could be detected again at the exact same locations. This indicates that the straining of dislocations does not lead to a new stable condition, but that dislocations creep in long time behaviour. Regarding long-term behaviour Davies and Bruce [10] found creep in nettle but not in flax fibres. Figure 5 shows exemplarily one of three fibres that were strained with a constant force for 20 min. During this short time span dislocations that had disappeared did not reappear. The constant stress could be retained by only slight adjustments of the force. Accordingly,the marginal relaxation of fibres indicates that the material is viscoelastic only to a small extent.
Images from polarized light microscopy of a hemp fibre before and at three different times after tensile testing (right after, 2 weeks after and 2 months after). The dislocations reappear after 2 months. Length of scalebar: 100 μm. The image taken 2 months after the failure shows the opposite site of the fibre, hence the difference in angle between the dislocations and the fibre longitudinal axis
The results of the present study can help understanding the results of some earlier studies. In a study on pulp fibres from Radiata pine, Kibblewhite [16] found that “zones of dislocations” (corresponding to the term dislocations used here) rapidly decreased by the beating process. This result could be a consequence of the same mechanism as seen here, i.e. that the dislocations were removed by tensile stress and that they did not reappear until after a rather long time period. Similarly, the result found in the present study might help explain the result of Ander et al. [17], who could not detect dislocations in spruce pulp fibres after tensile failure.
The disappearance of dislocations during tensile testing could help explain the results in Fig. 1, i.e. that the extent of dislocations in a hemp fibre does not appear to affect its mechanical properties. If both small and large dislocations are pulled straight during tensile testing as seen in Fig. 2, and if the stiffness of the fibre is only marginally affected by this alignment, then it is understandable that the extent of the dislocations has no effect on neither the ultimate tensile stress nor on the MOE. However, Davies and Bruce [10] might still be correct when they conclude that it is unlikely to find a strong fibre with many dislocations, as the likelihood of the fibre also having some weak point is higher than if it has no dislocations. One explanation could be that the presence of dislocations signals that the fibre has experienced stresses either before or after harvest. The severity of damage is thus not directly related to the number or size of dislocations induced during the same event, but the presence of many dislocations indicates that it is also likely that several weak points are present. From our data it seems reasonable to conclude that dislocations are pulled straight during tensile straining and thus, converge to the structure of the unaffected fibre segments. For this reason straightened dislocations may still function as weak points but rather close to stress levels that would cause the unaffected segments to fail. Accordingly, we speculate that the main reason for the large scatter in the mechanical properties of hemp fibres is due to the occurrence of inherently weaker and stronger fibres, for example due to the status of the plant during the time of formation of each particular fibre (e.g. plant age, availability of moisture, nutrients etc.) and that dislocations play a minor role.
Conclusions
The first part of this study comprised 53 single hemp fibres with up to 20% of their cell wall consisting of dislocations. For this data set the amount of dislocations did not affect the ultimate tensile stress of single hemp fibres or the MOE. In the second part of the study it was found that dislocations disappeared during tensile testing, and that they did not reappear until several weeks after failure. The straightening of the dislocations did not affect the stiffness of the fibres, it remained the same throughout the tensile test, i.e. there was no strain stiffening. Fracture surfaces of fibres indicate that the former positions of the dislocations may have functioned as locations for crack initiation, but the fibres broke in shear failure between microfibrils within the cell wall. We conclude that the large scatter in the mechanical properties is mainly due to the occurrence of inherently weaker and stronger fibres and only marginally affected by dislocations.
References
Nyholm K, Ander P, Bardage S, Daniel G (2001) Nordic Pulp Paper Res J 4:376
Hughes M, Sèbe G, Hague J, Hill C, Spear M, Mott L (2000) Composite Interfaces 7:13
Bos HL, Van Den Oever MJA, Peters OCJJ (2002) J Mater Sci 37:1683
Bos HL, Donald AM (1999) J Mater Sci 34:3029
Baley C (2004) J Mater Sci 39:331
Focher B, Marzetti A, Sharma HSS (1992) In: Sharma HSS, Van Sumere CF (eds) The biology and processing of flax, M. Publications, Belfast, p 333. Cited from [Hughes M, Sebe G, Hague J, Hill C, Spear M, Mott L (2000) Composite Interfaces 7:13]
Koch G, Bauch J, Dünisch O, Seehann G, Schmitt U (1996) Holz Roh Werkst 54:243
Robinon W (1920) Phil Trans R Soc B 210:49
Page DH, El-Hosseiny F, Winkler K, Bain R (1972) Pulp Paper Mag Can 73(8):72
Davies GC, Bruce DM (1998) Textile Res J 68:623
Burgert I, Frühmann K, Keckes J, Fratzl P, Stanzl-Tschegg SE (2003) Holzforschung 57:661
Robson D, Hague J (1995) In: Proceedings of the conference on woodfiber–plastic composites, (Forest Products Society), Madison, Wisconsin USA p 41
Groom L, Mott L, Shaler SM (2002) Wood Fiber Sci 34:14
Burgert I, Eder M, Frühmann K, Keckes J, Fratzl P, Stanzl-Tschegg SE (2005) Holzforschung 59:354
Thygesen A (2005) Optimisation and characterisation of hemp fibres needed to go from the plant to fibres in composite materials, Ph.D. Thesis, Risø National Laboratory, Denmark
Kibblewhite RP (1976) Cellulose Chem. Technol 10:497
Ander P, Burgert I, Frühmann K (2003) In: Salmén L (ed) Proceedings of the second international conference of the European society for wood mechanics (ESWM), May 25th–28th, Stockholm, Sweden, p 63
Acknowledgements
The present study was carried out within the project ‘High Performance Hemp Fibres and Improved Fibre Network for Composites’ financed by the Danish Research Council. LGT thanks COST Action E35 for financing a Short Term Scientific Mission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thygesen, L.G., Eder, M. & Burgert, I. Dislocations in single hemp fibres—investigations into the relationship of structural distortions and tensile properties at the cell wall level. J Mater Sci 42, 558–564 (2007). https://doi.org/10.1007/s10853-006-1113-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1113-5