Abstract
Novel wet-chemical methods of synthesis have been adopted to synthesize nano-crystalline CeO2 and Gd-substituted compositions aiming to explore an efficient oxide ion conducting solid electrolyte for intermediate temperature solid oxide fuel cell (IT-SOFC) applications. Nano-crystalline CeO2 powders were synthesized by combustion method using redox mixture of cerric ammonium nitrate or cerium nitrate and maleic acid or 1,3-dimethylurea and compared with high surface area CeO2 powders prepared by hydrothermal technique with microwave precipitated precursor from aqueous solutions of (NH4)2Ce(NO3)6 and urea. The grain size achieved by the hydrothermal technique is ∼7 nm which is smaller than that of commercial nano CeO2 powders. Conventional or microwave sintering was used to prepare dense Ce0.8Gd0.2O1.9 pellets from the ceria powders made of redox mixture of cerium nitrate, 1,3-dimethylurea (DMU) and Gd2O3 as the starting ingredients. The samples were characterized by X-ray diffractometry (XRD), transmission electron microscopy (TEM), diffuse reflectance spectroscopy (DRS), scanning electron microscopy (SEM), and ac impedance spectroscopy. The ionic conductivity measured for the pellet sintered at 1400 °C is 1 × 10−2 and 2.4 × 10−2 S/cm at 700 °C and 800 °C respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluorite structured Ceria (CeO2) has many applications as oxygen sensor [1], auto exhaust catalysts [2], polishing agents [3], sunscreens [4], and electrolyte materials for intermediate temperature solid oxide fuel cell (IT-SOFC) [5, 6]. Although yttria stabilized zirconia (YSZ) is still the favored electrolyte materials; ceria is considered alternative electrolyte material and could operate at lower temperatures (500–700 °C). The ionic conductivity of ceria is approximately an order of magnitude greater than that of YSZ for comparable doping condition and especially when its nano-crystalline nature is retained. However, CeO2 is not a pure ionic conductor but has electronic conduction too, at elevated temperatures and electronic conduction gets enhanced in reducing atmospheres. IT-SOFC stacks operating at reduced temperature offer many exiting advantages, for example, increased material durability and reduced overall cost. They allow the use of gaskets that will permit greater design flexibility for the stack configuration, and allow the metal-supported positive-electrolyte-negative (PEN) structures, which will withstand the rapid temperature cycles during operation of small stacks [7–9].
Ceria is highly refractory oxide and difficult to sinter without sintering aids. However, nano-crystalline ceria can be densified at much lower temperature because of high surface energy of the nanoparticles [10–13]. Many solution based techniques were used to obtain the nanocrystalline ceria powders, such as hydrothermal technique [14], mimic alkoxide technique [15], micro-emulsion technique [16], sol–gel technique [17], precipitation technique [18], glycine–nitrate combustion technique [19], hydrazine technique [20], and spray hydrolysis [21]. In the past, nanocrystalline CeO2 powders have been prepared by using urea and hexamethylenetetramine-based precipitation [17, 18]. In these experiments precipitation was carried out by conventional heating of the precursor solution at 80 °C. Since microwave heating is rapid and uniform, homogeneous nucleation and agglomerate-free precipitation is expected when the precursor solution is heated by microwaves. In this work emphasis has been given on the preparation of uniform nano-crystalline ceria powders at 200 °C, by hydrothermal treatment of microwave precipitated precursor from aqueous solution of (NH4)2Ce(NO3)6 and urea. Nanocrystalline CeO2 and Ce0.8Gd0.2O1.9 powders were also prepared by solution combustion technique using different kinds of starting reagents for comparison. The ceria powders prepared in this study were characterized by X-ray diffractometry (XRD), transmission electron microscopy (TEM), and diffuse reflectance spectroscopy (DRS), and compared with commercial nanocrystalline ceria powders. Combustion synthesized Ce0.8Gd0.2O1.9 powders were made to dense pellets by using a resistance-heating furnace at 1400 °C and 1550 °C; and microwave sintering at 1200 °C. The microstructure of the sintered pellets were examined by scanning electron microscopy (SEM), and their electrical conductivity was measured by ac impedance spectroscopy (EIS). The results of the experimental observations are discussed in this paper.
Experimental
Synthesis
In this study, CeO2 nanocrystalline powders were prepared by solution combustion and hydrothermal techniques,. Starting from four kinds of solution mixtures, the CeO2 powders were synthesized by the solution combustion route. The four kinds of starting solution were: (a) redox mixture of cerric ammonium nitrate (CAN) and maleic acid; (b) redox mixture of cerium nitrate and maleic acid; (c) redox mixture of cerric ammonium nitrate (CAN) and 1,3-dimethylurea (DMU); and (d) redox mixture of cerium nitrate and 1,3-dimethylurea (DMU). Assuming complete combustion of the redox mixture, the theoretical equation for the formation of CeO2 may be written as follows:
-
a)
(NH4)2Ce(NO3)6 + 2(HOOC-CH=CH-COOH) → CeO2 + 4N2 + 8CO2 + 8H2O;
-
b)
4Ce(NO3)3 + 5(HOOC-CH=CH-COOH) + O2 → 4CeO2 + 6N2 + 20CO2 + 10H2O;
-
c)
3(NH4)2Ce(NO3)6 + 4(H3C-NH-CO-NH-CH3) → 3CeO2 + 4N2 + 12CO2 + 28H2O;
-
d)
6Ce(NO3)3 + 5(H3C-NH-CO-NH-CH3) + 3/2O2→ 6CeO2 + 14N2 + 15CO2 + 20H2O.
A typical solution combustion process can be described considering the case (a) as given above as an example: 10 g of CAN were taken in a 250 mL petridish dissolved in minimum amount of water, 4.234 g of maleic acid was added to this solution. The redox mixture of CAN and maleic acid was slightly heated on the hot plate to completely dissolve the reactants. Then the petridish was introduced into a preheated furnace (600 °C). The solution boiled and frothed leading to self propagating-type combustion after a few seconds. The product was bright yellow in color. The details of the procedure are given in the flowchart (Fig. 1a).
The hydrothermal technique has more processing steps than the solution combustion technique. The aqueous solution of 10 g of (NH4)2Ce(NO3)6 and 16 g of urea was heated in a domestic microwave (2.45 GHz, 700Watts) at maximum power for 3 min. No turbidity was observed. When this solution was kept under ambient conditions in an open beaker without stirring for an hour, turbidity was observed. The solid settled down when the turbid solution was kept standing under ambient conditions in an open beaker without stirring overnight. The solution was centrifuged. The microwave reheating at maximum power of the transparent yellow supernatant in open beaker did not result in any significant turbidity. The pale yellow slimy residue obtained from centrifugation was transferred to a teflon container in a steel lined autoclave and hydrothermally treated at 200 °C for 48 h. The product obtained was spongy, swollen and voluminous. It easily separated from the teflon container. The product smelled of little ammonia. The product was rinsed with double distilled water and dried overnight in oven at 60 °C. The product obtained was shrunk in volume. It was ground and the color is lighter than the commercial nano-active CeO2. The detailed steps of the process are given in the flowchart (Fig. 1b). Gd-doped ceria was also prepared by solution combustion technique using redox mixture of cerium nitrate, 1,3-dimethylurea (DMU) and Gd2O3. The details are shown as a flowchart in Fig. 1(c). The ceria powders were pelletized under 230 MPa at room temperature into pellets of 1.2 mm thick and 11 mm diameter. The pellets were sintered in resistance heating furnace (Carbolite RHF 1600) at 1400 °C and 1550 °C for 12 h, or sintered in a microwave oven (Panasonic, 2.45 GHz, 1200 W) at 1200 C for 60 min. The microwave oven was equipped with a thermocouple and a programmable temperature controller (ThermWave Mod.III, Research Microwave Systems LLC).
Characterization
The powder and pellet samples were characterized by XRD, transmission electron microscopy (TEM), diffuse reflectance spectroscopy (DRS), scanning electron microscopy (SEM), and ac impedance spectroscopy (EIS). The XRD data were obtained at room temperature by a Siemens/Bruker D5000 diffractometer using CuKα as the incident X-ray. The TEM was taken by a JEOL 2010 High-resolution Transmission Electron Microscope (HRTEM) .The DRS was scanned by a CARY 500 Scan UV–Vis-NIR spectrophotometer. The SEM was taken by a JEOL 6300F microscope using 3–5 kV as the accelerating voltages of the electron beam. The EIS was measured by HP-4192A LF Impedance Analyzer (5–13 MHz) in air at temperature range of 200–900 °C. The two electrodes were formed by applying platinum paste to the two sides of the pellet and firing at temperature 980 °C for 30 min or silver paste firing at 500 °C for 30 min.
Results and discussion
XRD of the ceria powders and pellets
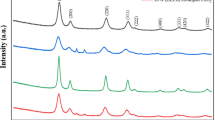
The X-ray diffraction of the ceria powders and pellets are shown in Fig. 2. The broad peaks in Fig. 2(a) indicate the ceria powders are nanocrystalline. The XRD pattern of CeO2 and Ce0.8Gd0.2O1.9 powders synthesized in this work (Fig. 2(a)) have quite similar XRD pattern as that of the commercial powders (NanoActive Cerium Oxide, NanoScale Materials, Inc.), this indicates that their crystal structure is same and their grain sizes are of same order of magnitude. The crystallite size of the particles were also measured by using Scherrer formula; t = 0.9λ/Bcosθ, Where, t = Average crystallite size, λ= wavelength of X-rays, θ = the position of the reflection in XRD pattern in degrees, B= integral breadth of a reflection (in radians 2θ) located at 2θ and often calculated by using a solid reference standard, i.e B2 = B 2s −B 2r . The particle size measured from TEM and XRD confirms the nano-crystalline nature of the particles. The XRD patterns of the ceria pellets prepared from the laboratory-made undoped and doped ceria powders are shown in Fig. 2(b). In Fig. 2(b) the peaks are very sharp indicating well crystalline nature of the material compared to those in Fig. 2(a) and there is no impurity peak shown except for the microwave-sintered pellet. The XRD data confirms that conventional (resistance heating) sintering produces well crystalline and phase pure CeO2 and Ce0.8Gd0.2O1.9 pellets.
TEM of the ceria powder
In Fig. 3(a–d) TEM and electron diffraction pattern of the ceria powders synthesized by different routes and different compositions has been shown. The undoped ceria in Fig. 3(a–c) have mean grain-size ∼5 nm, and the doped ceria in Fig. 3d has a bigger mean grain-size ∼20 nm. It is noticeable that the electron diffraction patterns in Fig. 3c are similar to the pattern of the commercial ceria powder in Fig. 3a. The similar smooth diffraction rings in Fig. 3c indicate that the ceria prepared in the laboratory through the hydrothermal synthesis has similar grain homogeneity as the commercial ones. The dark diffraction spots scattered along the rings in Fig. 3b and 3d reflect the presence of some big grain in the powders and the grain evenness is not as perfect as the ones in Fig. 3a and c.
Diffuse reflectance spectra (DRS) of the ceria powder
In Fig. 4 the diffuse reflectance spectra of the undoped and doped ceria synthesized through different routes has been shown. For ceria powders synthesized through the conventional solution route as shown in Fig. 1a exhibited similar DRS patterns as the commercial one, ref. Fig. 4I(a), (b), (c) and (e) for CeO2, and Fig. 4II(b) for Ce0.8Gd0.2O1.9. However, the powders synthesized through the hydrothermal route in Fig. 1c has an obvious blue shift comparing to the commercial one as shown in Fig. 4(I)-(d) and (f). The ultraviolet absorption is commonly believed a direct charge-transfer transition between O-2p and Ce-4f bands. Due to the quantum size effect, the blue shift is inversely proportional to the nearly square of the particle diameter [22]. In this case, the blue shift of the hydrothermally synthesized ceria in Fig. 4-I-(d) indicates that the ceria synthesized in this work has a smaller mean grain size than the commercial ceria in Fig. 4-I-(e).
SEM of the ceria pellets
Some pellets were made from the nano-crystalline ceria powders. The scanning electron microscopy pictures of the ceria pellets are shown in Fig. 5(a)–(d). Fig. 5(a) and (b) show the sintering temperature effect on the grain size of the doped ceria. The mean grain size of pellet sintered at temperature 1550 °C (∼7 μm) is about seven times bigger than the one sintered at 1400 °C (∼1 μm). We also sintered the pellet by using microwave, as shown in Fig. 5(c). The pellet was sintered under microwave at 1200 °C for 60 min. The mean grain size is about 0.2 μm, which is very small comparing to the upper two conventionally sintered pellets. We also made an undoped ceria pellet sintered at 1550 °C as shown in Fig. 5(d). It is noticeable that the grain size is very big, up to 100 μm. The mean grain size is about ten times bigger than that of the Gd-doped ceria pellet sintered at the same temperature (comparing Fig. 5(d) to 5(b)).
Impedance spectroscopy (EIS) of the ceria pellets
We measured the conductivities of the Gd-doped pellets by ac impedance spectroscopy in air. The transport number is nearly equal to one in air or an inert atmosphere [23]. The Arrhenius relations of conductivity versus temperature are shown in Fig. 6. All the three samples display similar conductivities and Arrhenius slope. Although the grain size of the microwave sintered ceria is as small as submicrometer, the sample (Fig. 6(c)) shows similar conductivity as the conventional sintered and bigger grained Ce0.8Gd0.2O1.9 (Fig. 6(a) and (b)). The sample has higher conductivity than the other two samples in the lower temperature region below 600 °C. The sample sintered at 1400 °C has a higher conductivity (1.0×10−2 S/cm at 700 °C and 2.4 × 10−2 S/cm at 800 °C) than the sample sintered at 1550 °C, even though the sample has smaller grain or bigger grain-boundary region. It shows the grain-boundary region has negligible contributions to the total resistance of these pellets.
Conclusion
Uniformly nano-grained ceria powders were synthesized at 200 °C by hydrothermal treatment of microwave precipitated precursor from aqueous solution of (NH4)2Ce(NO3)6 and urea. The average grain size (∼5 nm) of hydrothermally treated ceria powders is smaller than that of commercial nano-grained ceria (∼7 nm) evident from the TEM and DRS studies. The obvious blue-shift in the DRS plot is believed to be caused by smaller nanoscale grain size.
Dense CeO2 and Ce0.8Gd0.2O1.9 pellets were prepared from the nanocrystalline powders. The pellets sintered by conventional furnace (resistance heating) at 1400 °C and 1500 °C were well-crystallized phase pure. The sample sintered at 1400 °C has ionic conductivities of 1.0 × 10−2 S/cm at 700 °C and 2.4 × 10−2 S/cm at 800 °C. The pellets sintered by microwave oven at 1200 °C have submicrometer grain size and secondary phase, which needs higher sintering temperature to achieve better crystallization.
Reference
Izu N, Shin W, Murayarna N, Kanzaki S (2002) Sens Actuators B87:95
Trovarelli A, (2002) Catalysis by Ceria and related materials. Imperial College Press, London
Hedrick JB, Sinha SP (1994) J Alloys Compd 207–208:377
Yabe S, Yamashita M, Momose S, Tahira K, Yoshida S, Li S, Yin S, Sato T (2001) Int J Inorg Mater 3:1003
Steele BCH (2000) Solid State Ionics 129:95
Hormes J, Pantelouris M, Balazs GB, Rambabu B (2000) Solid State Ionics 136–137:954
Brandon NP, Skinner S, Steele BCH (2003) Annu Rev Mater Res 33:183
Goodenough JB (2003) Ann Rev Mater Res 33:91
Haile SM (2003) Acta Mater 51:5981
Tuller HL (2000) Solid State Ionics 131:143
Chiang YM, Lavik EB, Kosacki I, Tuller HL, Ying JY (1996) Appl Phys Lett 69:185
Tschope A, Ying JY, Tuller HL (1996) Sens Actuators B31:111
Basu S, Davi PS, Maiti HS (2004) J Mater Res 19:3162
Hirano M, Kato E (1996) J Mater Sci Lett 15:1249
Li JG, Ikegami T, Lee JH, Mori T (2001) Acta Mater 49:419
Zhang J, Ju X, Wu ZY, Liu T, Hu TD, Xie YN, Zhang ZL (2001) Chem Mater 13:4192
Chu X, Chung W, Schmidt LD (1993) J Am Ceram Soc 76:2115
Chen PL, Chen IW (1993) J Am Ceram Soc 76:1577
Purohit RD, Sharma BP, Pillai KT, Tyagi AK (2001) Mater Res Bull 36:2711
Nakane S, Tachi T, Yoshinaka M, Hirota K, Yamaguchi O (1997) J Am Ceram Soc 80:3221
Xu H, Gao L, Gu H, Yan D (2002) J Am Ceram Soc 85:139
Tsunekawa S, Fukuda T (2000) J Appl Phys 87:1318
Huang K, Feng M, Goodenough JB (1998) J Am Ceram Soc 81:357
Acknowledgements
BRB acknowledge the U.S-DOE-NETL for supporting this work (grant #DE-FG-26NT41915). S. Ghosh and H. Jena thank SUBR for availing exchange visitor assignment in Solid State Ionics Labaratory (BRB). H. Jena acknowledge IGCAR (Govt. of India) for granting EOL to avail this opportunity in USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rambabu, B., Ghosh, S. & Jena, H. Novel wet-chemical synthesis and characterization of nanocrystalline CeO2 and Ce0.8Gd0.2O1.9 as solid electrolyte for intermediate temperature solid oxide fuel cell (IT-SOFC) applications. J Mater Sci 41, 7530–7536 (2006). https://doi.org/10.1007/s10853-006-0837-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0837-6